Functional rescue in an Angelman syndrome model following treatment with lentivector transduced hematopoietic stem cells
- PMID: 33856035
- PMCID: PMC8188406
- DOI: 10.1093/hmg/ddab104
Functional rescue in an Angelman syndrome model following treatment with lentivector transduced hematopoietic stem cells
Abstract
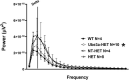
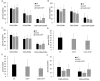
Angelman syndrome (AS) is a rare neurodevelopmental disorder characterized by impaired communication skills, ataxia, motor and balance deficits, intellectual disabilities, and seizures. The genetic cause of AS is the neuronal loss of UBE3A expression in the brain. A novel approach, described here, is a stem cell gene therapy which uses lentivector-transduced hematopoietic stem and progenitor cells to deliver functional UBE3A to affected cells. We have demonstrated both the prevention and reversal of AS phenotypes upon transplantation and engraftment of human CD34+ cells transduced with a Ube3a lentivector in a novel immunodeficient Ube3amat-/pat+ IL2rg-/y mouse model of AS. A significant improvement in motor and cognitive behavioral assays as well as normalized delta power measured by electroencephalogram was observed in neonates and adults transplanted with the gene modified cells. Human hematopoietic profiles observed in the lymphoid organs by detection of human immune cells were normal. Expression of UBE3A was detected in the brains of the adult treatment group following immunohistochemical staining illustrating engraftment of the gene-modified cells expressing UBE3A in the brain. As demonstrated with our data, this stem cell gene therapy approach offers a promising treatment strategy for AS, not requiring a critical treatment window.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Williams, C.A. (2005) Neurological aspects of the Angelman syndrome. Brain Dev., 27, 88–94. - PubMed
-
- Buiting, K., Williams, C. and Horsthemke, B. (2016) Angelman syndrome-insights into a rare neurogenetic disorder. Nat. Rev. Neurol., 12, 584–593. - PubMed
-
- Williams, C.A., Driscoll, D.J. and Dagli, A.I. (2010) Clinical and genetic aspects of Angelman syndrome. Genet. Med., 12, 385–395. - PubMed
-
- Chamberlain, S.J. and Lalande, M. (2010) Neurodevelopmental disorders involving genomic imprinting at human chromosome 15q11-q13. Neurobiol. Dis., 39, 13–20. - PubMed
-
- Matsuura, T., Sutcliffe, J.S., Fang, P., Galjaard, R.J., Jiang, Y.H., Benton, C.S., Rommens, J.M. and Beaudet, A.L. (1997) De novo truncating mutations in E6-AP ubiquiting-protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet., 15, 74–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

