Photochemical Regioselective C(sp3)-H Amination of Amides Using N-Haloimides
- PMID: 33856220
- PMCID: PMC9652782
- DOI: 10.1021/acs.orglett.1c00831
Photochemical Regioselective C(sp3)-H Amination of Amides Using N-Haloimides
Abstract
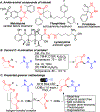
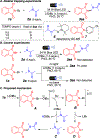
A metal-free regioselective C(sp3)-H amination of amides using N-haloimides in the presence of lithium tert-butoxide and visible light is presented herein. This photoexcited approach is straightforward, and it aminates a wide variety of amides under mild conditions without the use of photocatalysts, external radical initiators, or oxidants. A halogen-bonded intermediate between the tert-butoxide base and the N-haloimide is proposed to be responsible for the increased photoreactivity. Calculations show that the formation of this electron donor-acceptor complex presents an exergonic energy profile.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Fischer J; Ganellin CR Analogue-based Drug Discovery; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; p 457.
- Van Epen JH Experience with Fluspirilene (R 6218), a Long Acting Neuroleptic. Psychiatr., Neurol., Neurochir 1970, 73, 277–284. - PubMed
- Taylor-Robinson D; Furr PM Observations on the Antibiotic Treatment of Experimentally Induced Mycoplasmal Infections in Mice. J. Antimicrob. Chemother 2000, 45, 903–907. - PubMed
- Pellock JM Treatment of Epilepsy in the New Millennium. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2000, 20, 129S–138S. - PubMed
-
- Anisimova NA; Belavin IY; Orlova NA; Sergeev VN; Shipov AG; Baukov YI Silyl Variation of N-Amidoalkylation. Zhurnal Obshchei Khimii 1983, 53, 1198–1199.
- Hellmann H; Lösehmann I; Lingens F Über N-Mannich-Basen, II. Mitteil.: Synthesen mit Dialkylamino-methyl-phthalimiden und deren quartären Salzen. Chem. Ber 1954, 87, 1690–1699.
-
-
For the only known examples of C–H amination to generate amido aminals, see:
- Sun M; Zhang T; Bao W FeCl3 Catalyzed sp3 C–H Amination: Synthesis of Aminals with Arylamines and Amides. Tetrahedron Lett. 2014, 55, 893–896.
- Xia Q; Chen W Iron-Catalyzed N-Alkylation of Azoles via Cleavage of an sp3 C–H Bond Adjacent to a Nitrogen Atom. J. Org. Chem 2012, 77, 9366–9373. - PubMed
- Saidulu G; Kumar RA; Reddy KR Iron-Catalyzed C–N Bond Formation via Oxidative Csp3–H Bond Functionalization Adjacent to Nitrogen in Amides and Anilines: Synthesis of N-Alkyl and N-Benzyl Azoles. Tetrahedron Lett. 2015, 56, 4200–4203.
- Deng X; Lei X; Nie G; Jia L; Li Y; Chen Y Copper Catalyzed Cross-Dehydrogenative N2-Coupling of NH-1,2,3-Triazoles with N,N-Dialkylamides: N-Amidoalkylation of NH-1,2,3-Triazoles. J. Org. Chem 2017, 82, 6163–6171. - PubMed
- Xu S; Luo Z; Jiang Z; Lin D Transition-Metal-Free N9-Amidoalkylation of Purines with N,N-Dialkylamides. Synlett 2017, 28, 868–872.
- Aruri H; Singh U; Kumar M; Sharma S; Aithagani SK; Gupta VK; Mignani S; Vishwakarma RA; Singh PP Metal-free Cross-Dehydrogenative Coupling of HN-Azoles with α-C(sp3)-H Amides via C-H Activation and Its Mechanistic and Application Studies. J. Org. Chem 2017, 82, 1000–1012. - PubMed
- Zhu Z; Wang Y; Yang M; Huang L; Gong J; Guo S; Cai H A Metal-Free Cross-Dehydrogenative Coupling Reaction of Amides to Access N-Alkylazoles. Synlett 2016, 27, 2705–2708.
- Lao Z-Q; Zhong W-H; Lou Q-H; Li Z-J; Meng X-B KI-Catalyzed Imidation of sp3 C–H Bond Adjacent to Amide Nitrogen Atom. Org. Biomol. Chem 2012, 10, 7869–7871. - PubMed
- Wan Z; Wang D; Yang Z; Zhang H; Wang S; Lei A Electrochemical Oxidative C(sp3)-H Azolation of Lactams Under Mild Conditions. Green Chem. 2020, 22, 3742–3747.
-
-
-
For selected reviews of photochemistry, see:
- Ghosh I; Marzo L; Das A; Shaikh R; König B Visible Light Mediated Photoredox Catalytic Arylation Reactions. Acc. Chem. Res 2016, 49, 1566–1577. - PubMed
- Tucker JW; Stephenson CRJ Shining Light on Photoredox Catalysis: Theory and Synthetic Applications. J. Org. Chem 2012, 77, 1617–1622. - PubMed
- Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322–5363. - PMC - PubMed
- Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074. - PMC - PubMed
- Le C; Liang Y; Evans RW; Li X; MacMillan DWC Selective sp3 C–H alkylation via polarity-match based cross-coupling. Nature 2017, 547, 79–83. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

