COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021
- PMID: 33856242
- PMCID: PMC8114441
- DOI: 10.1177/13524585211003476
COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021
Abstract
Background: Since vaccination against coronavirus disease 2019 (COVID-19) became available, risks related to vaccinating patients with multiple sclerosis (MS) need to be carefully assessed.
Objective: Characterize safety and occurrence of immediate relapses following COVID-19 vaccination in a large cohort of MS patients.
Methods: We assessed the safety of BNT162b2 COVID-19 vaccination in adult MS patients.
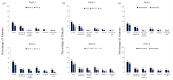
Results: Between 20 December 2020 and 25 January 2021, 555 MS patients received the first dose of BNT162b2 vaccine and 435 received the second dose. There were three cases of COVID-19 infection encountered after the first dose. Safety profile of COVID-19 vaccine was characterized by pain at the injection site, fatigue, and headache. No increased risk of relapse activity was noted over a median follow-up of 20 and 38 days after first and second vaccine doses, respectively. The rate of patients with acute relapse was 2.1% and 1.6% following the first and second doses, respectively, similar to the rate in non-vaccinating patients during the corresponding period. Mild increase in the rate of adverse events was noted in younger patients (18-55 years), among patients with lower disability (Expanded Disability Status Scale (EDSS) ⩽3.0), and in patients treated with immunomodulatory drugs.
Conclusion: COVID-19 BNT162b2 vaccine proved safe for MS patients. No increased risk of relapse activity was noted.
Keywords: COVID-19; Multiple sclerosis; acute relapse; adverse events; immune response; vaccination.
Conflict of interest statement
Figures
References
-
- Chen RT, Pless R, Destefano F. Epidemiology of autoimmune reactions induced by vaccination. J Autoimmun 2001; 16(3): 309–318. - PubMed
-
- Kivity S, Agmon-Levin N, Blank M, et al.. Infections and autoimmunity: Friends or foes? Trends Immunol 2009; 30: 409–414. - PubMed
-
- Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol 2011; 68(10): 1267–1271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical