Use of Endoscopic Images in the Prediction of Submucosal Invasion of Gastric Neoplasms: Automated Deep Learning Model Development and Usability Study
- PMID: 33856356
- PMCID: PMC8085753
- DOI: 10.2196/25167
Use of Endoscopic Images in the Prediction of Submucosal Invasion of Gastric Neoplasms: Automated Deep Learning Model Development and Usability Study
Abstract
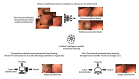
Background: In a previous study, we examined the use of deep learning models to classify the invasion depth (mucosa-confined versus submucosa-invaded) of gastric neoplasms using endoscopic images. The external test accuracy reached 77.3%. However, model establishment is labor intense, requiring high performance. Automated deep learning (AutoDL) models, which enable fast searching of optimal neural architectures and hyperparameters without complex coding, have been developed.
Objective: The objective of this study was to establish AutoDL models to classify the invasion depth of gastric neoplasms. Additionally, endoscopist-artificial intelligence interactions were explored.
Methods: The same 2899 endoscopic images that were employed to establish the previous model were used. A prospective multicenter validation using 206 and 1597 novel images was conducted. The primary outcome was external test accuracy. Neuro-T, Create ML Image Classifier, and AutoML Vision were used in establishing the models. Three doctors with different levels of endoscopy expertise were asked to classify the invasion depth of gastric neoplasms for each image without AutoDL support, with faulty AutoDL support, and with best performance AutoDL support in sequence.
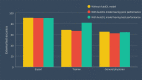
Results: The Neuro-T-based model reached 89.3% (95% CI 85.1%-93.5%) external test accuracy. For the model establishment time, Create ML Image Classifier showed the fastest time of 13 minutes while reaching 82.0% (95% CI 76.8%-87.2%) external test accuracy. While the expert endoscopist's decisions were not influenced by AutoDL, the faulty AutoDL misled the endoscopy trainee and the general physician. However, this was corrected by the support of the best performance AutoDL model. The trainee gained the most benefit from the AutoDL support.
Conclusions: AutoDL is deemed useful for the on-site establishment of customized deep learning models. An inexperienced endoscopist with at least a certain level of expertise can benefit from AutoDL support.
Keywords: artificial intelligence; automated deep learning; convolutional neural network; deep learning; deep learning model; endoscopy; gastric neoplasms; neural network.
©Chang Seok Bang, Hyun Lim, Hae Min Jeong, Sung Hyeon Hwang. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 15.04.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



Similar articles
-
Deep learning-based clinical decision support system for gastric neoplasms in real-time endoscopy: development and validation study.Endoscopy. 2023 Aug;55(8):701-708. doi: 10.1055/a-2031-0691. Epub 2023 Feb 8. Endoscopy. 2023. PMID: 36754065 Clinical Trial.
-
Automated classification of gastric neoplasms in endoscopic images using a convolutional neural network.Endoscopy. 2019 Dec;51(12):1121-1129. doi: 10.1055/a-0981-6133. Epub 2019 Aug 23. Endoscopy. 2019. PMID: 31443108
-
Prediction of Submucosal Invasion for Gastric Neoplasms in Endoscopic Images Using Deep-Learning.J Clin Med. 2020 Jun 15;9(6):1858. doi: 10.3390/jcm9061858. J Clin Med. 2020. PMID: 32549190 Free PMC article.
-
Usefulness of artificial intelligence in gastric neoplasms.World J Gastroenterol. 2021 Jun 28;27(24):3543-3555. doi: 10.3748/wjg.v27.i24.3543. World J Gastroenterol. 2021. PMID: 34239268 Free PMC article. Review.
-
Artificial intelligence for gastric cancer in endoscopy: From diagnostic reasoning to market.Dig Liver Dis. 2024 Jul;56(7):1156-1163. doi: 10.1016/j.dld.2024.04.019. Epub 2024 May 18. Dig Liver Dis. 2024. PMID: 38763796 Review.
Cited by
-
Impact of the Volume and Distribution of Training Datasets in the Development of Deep-Learning Models for the Diagnosis of Colorectal Polyps in Endoscopy Images.J Pers Med. 2022 Aug 24;12(9):1361. doi: 10.3390/jpm12091361. J Pers Med. 2022. PMID: 36143146 Free PMC article.
-
Deep Learning and Gastric Cancer: Systematic Review of AI-Assisted Endoscopy.Diagnostics (Basel). 2023 Dec 6;13(24):3613. doi: 10.3390/diagnostics13243613. Diagnostics (Basel). 2023. PMID: 38132197 Free PMC article. Review.
-
Computer-Aided Diagnosis of Gastrointestinal Protruded Lesions Using Wireless Capsule Endoscopy: A Systematic Review and Diagnostic Test Accuracy Meta-Analysis.J Pers Med. 2022 Apr 17;12(4):644. doi: 10.3390/jpm12040644. J Pers Med. 2022. PMID: 35455760 Free PMC article. Review.
-
Automatic Classification of GI Organs in Wireless Capsule Endoscopy Using a No-Code Platform-Based Deep Learning Model.Diagnostics (Basel). 2023 Apr 11;13(8):1389. doi: 10.3390/diagnostics13081389. Diagnostics (Basel). 2023. PMID: 37189489 Free PMC article.
-
Edge Artificial Intelligence Device in Real-Time Endoscopy for Classification of Gastric Neoplasms: Development and Validation Study.Biomimetics (Basel). 2024 Dec 22;9(12):783. doi: 10.3390/biomimetics9120783. Biomimetics (Basel). 2024. PMID: 39727787 Free PMC article.
References
-
- Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019 Apr 14;25(14):1666–1683. doi: 10.3748/wjg.v25.i14.1666. https://www.wjgnet.com/1007-9327/full/v25/i14/1666.htm - DOI - PMC - PubMed
-
- Bang CS. [Deep Learning in Upper Gastrointestinal Disorders: Status and Future Perspectives] Korean J Gastroenterol. 2020 Mar 25;75(3):120–131. doi: 10.4166/kjg.2020.75.3.120. http://www.kjg.or.kr/journal/view.html?doi=10.4166/kjg.2020.75.3.120 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

