Development and Evaluation of a Method to Correct Misinterpretation of Clinical Trial Results With Long-term Survival
- PMID: 33856410
- PMCID: PMC8050786
- DOI: 10.1001/jamaoncol.2021.0289
Development and Evaluation of a Method to Correct Misinterpretation of Clinical Trial Results With Long-term Survival
Abstract
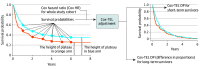
Importance: In immune checkpoint inhibitor (ICI) trials, long tails and crossovers in survival curves-which violate the proportional hazards (PH) assumption-are commonly observed, making cure or restricted mean survival time models preferable for analysis of ICI survival data. Cox PH analysis, however, still appears in major medical journals, leading to potential misinterpretation of clinical significance.
Objective: To convert inappropriate Cox hazard ratios (HRs) to appropriate PH cure model treatment-effect estimates (HR for short-term survivors and difference in proportions [DP] for long-term survivors) for more accurate interpretation of published ICI trials.
Design and setting: This study uses the Taylor expansion technique to demonstrate the mathematical relationship between Cox PH and PH cure models for data with long-term survival, and based on this relationship, proposes the Cox-TEL (Cox PH-Taylor expansion adjustment for long-term survival data) adjustment method. The proposed Cox-TEL method requires only 2 inputs: the reported Cox HRs and Kaplan-Meier-estimated survival probabilities.
Results: Comprehensive simulations show the strength of the proposed method in terms of power, bias, and type I error rate; these results, which are close to PH cure model estimates, were further verified in a melanoma data set (N = 285; Cox HR = 0.71; 95% CI, 0.51-0.91; Cox-TEL HR = 0.83; 95% CI, 0.60-1.07; PH cure HR = 0.86; 95% CI, 0.61-1.11; Cox-TEL DP = 0.10; 95% CI, 0.01-0.23; PH cure DP = 0.10; 95% CI, 0.00-0.21). The magnitude of potential difference between reported and adjusted HRs using real-world ICI trial results is demonstrated. For example, in the CheckMate 067 trial (nivolumab/ipilimumab combination therapy vs ipilimumab), the Cox HR was 0.54 (95% CI, 0.44-0.67), and the Cox-TEL HR was 0.90 (95% CI, 0.73-1.11).
Conclusions and relevance: The findings of this study suggest the need to revisit published ICI survival data analysis to address potential misinterpretation. The Cox-TEL method not only is designed for this purpose, but also is user friendly and easy to implement using published clinical trial data and a freely available R software package.
Conflict of interest statement
Figures

References
-
- Kuk AYC, Chen C-H. A mixture model combining logistic regression with proportional hazards regression. Biometrika. 1992; 79(3): 531-541. doi: 10.1093/biomet/79.3.531 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

