Machine learning based on clinical characteristics and chest CT quantitative measurements for prediction of adverse clinical outcomes in hospitalized patients with COVID-19
- PMID: 33856514
- PMCID: PMC8046645
- DOI: 10.1007/s00330-021-07957-z
Machine learning based on clinical characteristics and chest CT quantitative measurements for prediction of adverse clinical outcomes in hospitalized patients with COVID-19
Abstract
Objectives: To develop and validate a machine learning model for the prediction of adverse outcomes in hospitalized patients with COVID-19.
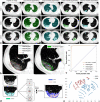
Methods: We included 424 patients with non-severe COVID-19 on admission from January 17, 2020, to February 17, 2020, in the primary cohort of this retrospective multicenter study. The extent of lung involvement was quantified on chest CT images by a deep learning-based framework. The composite endpoint was the occurrence of severe or critical COVID-19 or death during hospitalization. The optimal machine learning classifier and feature subset were selected for model construction. The performance was further tested in an external validation cohort consisting of 98 patients.
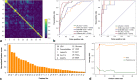
Results: There was no significant difference in the prevalence of adverse outcomes (8.7% vs. 8.2%, p = 0.858) between the primary and validation cohorts. The machine learning method extreme gradient boosting (XGBoost) and optimal feature subset including lactic dehydrogenase (LDH), presence of comorbidity, CT lesion ratio (lesion%), and hypersensitive cardiac troponin I (hs-cTnI) were selected for model construction. The XGBoost classifier based on the optimal feature subset performed well for the prediction of developing adverse outcomes in the primary and validation cohorts, with AUCs of 0.959 (95% confidence interval [CI]: 0.936-0.976) and 0.953 (95% CI: 0.891-0.986), respectively. Furthermore, the XGBoost classifier also showed clinical usefulness.
Conclusions: We presented a machine learning model that could be effectively used as a predictor of adverse outcomes in hospitalized patients with COVID-19, opening up the possibility for patient stratification and treatment allocation.
Key points: • Developing an individually prognostic model for COVID-19 has the potential to allow efficient allocation of medical resources. • We proposed a deep learning-based framework for accurate lung involvement quantification on chest CT images. • Machine learning based on clinical and CT variables can facilitate the prediction of adverse outcomes of COVID-19.
Keywords: Artificial intelligence; COVID-19; Prognosis; Tomography, X-ray computed.
© 2021. European Society of Radiology.
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

