Consensus on Treatment and Follow-Up for Biochemical Recurrence in Castration-Sensitive Prostate Cancer: A Report From the First Global Prostate Cancer Consensus Conference for Developing Countries
- PMID: 33856897
- PMCID: PMC8162965
- DOI: 10.1200/GO.20.00508
Consensus on Treatment and Follow-Up for Biochemical Recurrence in Castration-Sensitive Prostate Cancer: A Report From the First Global Prostate Cancer Consensus Conference for Developing Countries
Abstract
Purpose: To present a summary of the treatment and follow-up recommendations for the biochemical recurrence in castration-sensitive prostate cancer (PCa) acquired through a questionnaire administered to 99 PCa experts from developing countries during the Prostate Cancer Consensus Conference for Developing Countries.
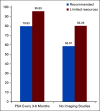
Methods: A total of 27 questions were identified as related to this topic from more than 300 questions. The clinician's responses were tallied and presented in a percentage format. Topics included the use of imaging for staging biochemical recurrence, treatment recommendations for three different clinical scenarios, the field of radiation recommended, and follow-up. Each question had 5-7 relevant response options, including "abstain" and/or "unqualified to answer," and investigated not only recommendations but also if a limitation in resources would change the recommendation.
Results: For most questions, a clear majority (> 50%) of clinicians agreed on a recommended treatment for imaging, treatment scenarios, and follow-up, although only a few topics reached a consensus > 75%. Limited resources did affect several areas of treatment, although in many cases, they reinforced more stringent criteria for treatment such as prostate-specific antigen values > 0.2 ng/mL and STAMPEDE inclusion criteria as a basis for recommending treatment.
Conclusion: A majority of clinicians working in developing countries with limited resources use similar cutoff points and selection criteria to manage patients treated for biochemically recurrent castration-sensitive PCa.
Conflict of interest statement
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 - PubMed
-
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent Eur Urol 71618–6292017 - PubMed
-
- Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial Eur Urol 63101–1072013 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

