Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer
- PMID: 33857345
- PMCID: PMC9425733
- DOI: 10.1002/chem.202100438
Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer
Abstract
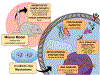
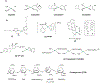
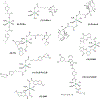
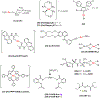
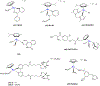
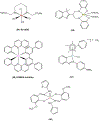
This review focuses on studies of coordination and organometallic compounds as potential chemotherapeutics against triple negative breast cancer (TNBC) which has one of the poorest prognoses and worst survival rates from all breast cancer types. At present, chemotherapy is still the standard of care for TNBC since only one type of targeted therapy has been recently developed. References for metal-based compounds studied in TNBC cell lines will be listed, and those of metal-specific reviews, but a detailed overview will also be provided on compounds studied in vivo (mostly in mice models) and those compounds for which some preliminary mechanistic data was obtained (in TNBC cell lines and tumors) and/or for which bioactive ligands have been used. The main goal of this review is to highlight the most promising metal-based compounds with potential as chemotherapeutic agents in TNBC.
Keywords: inorganic chemistry; mechanisms; medicinal chemistry; metallodrugs; triple negative breast cancer (TNBC).
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures




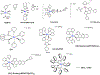


Similar articles
-
Therapeutic potential of ERK5 targeting in triple negative breast cancer.Oncotarget. 2014 Nov 30;5(22):11308-18. doi: 10.18632/oncotarget.2324. Oncotarget. 2014. PMID: 25350956 Free PMC article.
-
Half-sandwich Os(ii) and Ru(ii) bathophenanthroline complexes: anticancer drug candidates with unusual potency and a cellular activity profile in highly invasive triple-negative breast cancer cells.Dalton Trans. 2018 Sep 11;47(35):12197-12208. doi: 10.1039/c8dt02236d. Dalton Trans. 2018. PMID: 30112527
-
A self-assembly nano-prodrug for triple-negative breast cancer combined treatment by ferroptosis therapy and chemotherapy.Acta Biomater. 2023 Mar 15;159:275-288. doi: 10.1016/j.actbio.2023.01.050. Epub 2023 Jan 26. Acta Biomater. 2023. PMID: 36709836
-
New strategies for triple-negative breast cancer--deciphering the heterogeneity.Clin Cancer Res. 2014 Feb 15;20(4):782-90. doi: 10.1158/1078-0432.CCR-13-0583. Clin Cancer Res. 2014. PMID: 24536073 Free PMC article. Review.
-
Targeted Therapies for Triple-Negative Breast Cancer.Curr Treat Options Oncol. 2019 Nov 21;20(11):82. doi: 10.1007/s11864-019-0682-x. Curr Treat Options Oncol. 2019. PMID: 31754897 Review.
Cited by
-
G2/M-Phase-Inhibitory Mitochondrial-Depolarizing Re(I)/Ru(II)/Ir(III)-2,2'-Bipyrimidine-Based Heterobimetallic Luminescent Complexes: An Assessment of In Vitro Antiproliferative Activity and Bioimaging for Targeted Therapy toward Human TNBC Cells.ACS Omega. 2023 Mar 21;8(13):12283-12297. doi: 10.1021/acsomega.2c08285. eCollection 2023 Apr 4. ACS Omega. 2023. PMID: 37033791 Free PMC article.
-
Synthesis, Structure, and Stability of Copper(II) Complexes Containing Imidazoline-Phthalazine Ligands with Potential Anticancer Activity.Pharmaceuticals (Basel). 2025 Mar 6;18(3):375. doi: 10.3390/ph18030375. Pharmaceuticals (Basel). 2025. PMID: 40143151 Free PMC article.
-
Impact of the Pd2Spm (Spermine) Complex on the Metabolism of Triple-Negative Breast Cancer Tumors of a Xenograft Mouse Model.Int J Mol Sci. 2021 Oct 5;22(19):10775. doi: 10.3390/ijms221910775. Int J Mol Sci. 2021. PMID: 34639114 Free PMC article.
-
Development of immunoliposomes containing cytotoxic gold payloads against HER2-positive breast cancers.RSC Med Chem. 2023 Nov 21;15(1):139-150. doi: 10.1039/d3md00334e. eCollection 2024 Jan 25. RSC Med Chem. 2023. PMID: 38283233 Free PMC article.
-
Intracellular Localization Studies of the Luminescent Analogue of an Anticancer Ruthenium Iminophosphorane with High Efficacy in a Triple-Negative Breast Cancer Mouse Model.Inorg Chem. 2021 Dec 20;60(24):19152-19164. doi: 10.1021/acs.inorgchem.1c02929. Epub 2021 Nov 30. Inorg Chem. 2021. PMID: 34846878 Free PMC article.
References
-
- Li CI, Malone KE, Daling JR, Arch. Intern. Med 2003, 163, 49–56. - PubMed
-
- Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC, Jemal A, Breast Cancer Res. Treat 2014, 145, 753–63. - PubMed
-
- Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D, Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, Mohar A, Arrieta Ó, Cancer 2011, 117, 3658–3669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

