DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network
- PMID: 33857403
- PMCID: PMC8221569
- DOI: 10.1016/j.molcel.2021.03.041
DNAJC9 integrates heat shock molecular chaperones into the histone chaperone network
Abstract
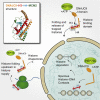
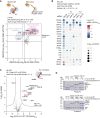
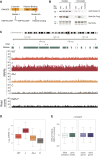
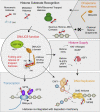
From biosynthesis to assembly into nucleosomes, histones are handed through a cascade of histone chaperones, which shield histones from non-specific interactions. Whether mechanisms exist to safeguard the histone fold during histone chaperone handover events or to release trapped intermediates is unclear. Using structure-guided and functional proteomics, we identify and characterize a histone chaperone function of DNAJC9, a heat shock co-chaperone that promotes HSP70-mediated catalysis. We elucidate the structure of DNAJC9, in a histone H3-H4 co-chaperone complex with MCM2, revealing how this dual histone and heat shock co-chaperone binds histone substrates. We show that DNAJC9 recruits HSP70-type enzymes via its J domain to fold histone H3-H4 substrates: upstream in the histone supply chain, during replication- and transcription-coupled nucleosome assembly, and to clean up spurious interactions. With its dual functionality, DNAJC9 integrates ATP-resourced protein folding into the histone supply pathway to resolve aberrant intermediates throughout the dynamic lives of histones.
Keywords: DNAJC9; HSP40; HSP70; MCM2; TONSL; chromatin replication; heat shock co-chaperone; histone chaperone; nucleosome assembly; transcription.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.M.H., D.J.P., H.H., and A.G. are inventors on a filed patent application covering the therapeutic targeting of TONSL for cancer therapy. A.G. is a co-founder and chief scientific officer (CSO) of Ankrin Therapeutics. A.G. is a member of Molecular Cell’s scientific advisory board.
Figures




Comment in
-
An energetic meet-and-greet: Molecular chaperones in the histone supply and deposition pathways.Mol Cell. 2021 Jun 17;81(12):2499-2501. doi: 10.1016/j.molcel.2021.05.012. Mol Cell. 2021. PMID: 34143966
References
-
- Alekseev O.M., Widgren E.E., Richardson R.T., O’Rand M.G. Association of NASP with HSP90 in mouse spermatogenic cells: stimulation of ATPase activity and transport of linker histones into nuclei. J. Biol. Chem. 2005;280:2904–2911. - PubMed
-
- Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

