Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis
- PMID: 33857405
- PMCID: PMC8041361
- DOI: 10.1016/S1473-3099(21)00146-8
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis
Abstract
Background: The comparative performance of different clinical sampling methods for diagnosis of SARS-CoV-2 infection by RT-PCR among populations with suspected infection remains unclear. This meta-analysis aims to systematically compare the diagnostic performance of different clinical specimen collection methods.
Methods: In this systematic review and meta-analysis, we systematically searched PubMed, Embase, MEDLINE, Web of Science, medRxiv, bioRxiv, SSRN, and Research Square from Jan 1, 2000, to Nov 16, 2020. We included original clinical studies that examined the performance of nasopharyngeal swabs and any additional respiratory specimens for the diagnosis of SARS-CoV-2 infection among individuals presenting in ambulatory care. Studies without data on paired samples, or those that only examined different samples from confirmed SARS-CoV-2 cases were not useful for examining diagnostic performance of a test and were excluded. Diagnostic performance, including sensitivity, specificity, positive predictive value, and negative predictive value, was examined using random effects models and double arcsine transformation.
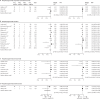
Findings: Of the 5577 studies identified in our search, 23 studies including 7973 participants with 16 762 respiratory samples were included. Respiratory specimens examined in these studies included 7973 nasopharyngeal swabs, 1622 nasal swabs, 6110 saliva samples, 338 throat swabs, and 719 pooled nasal and throat swabs. Using nasopharyngeal swabs as the gold standard, pooled nasal and throat swabs gave the highest sensitivity of 97% (95% CI 93-100), whereas lower sensitivities were achieved by saliva (85%, 75-93) and nasal swabs (86%, 77-93) and a much lower sensitivity by throat swabs (68%, 35-94). A comparably high positive predictive value was obtained by pooled nasal and throat (97%, 90-100) and nasal swabs (96%, 87-100) and a slightly lower positive predictive value by saliva (93%, 88-97). Throat swabs have the lowest positive predictive value of 75% (95% CI 45-96). Comparably high specificities (range 97-99%) and negative predictive value (range 95-99%) were observed among different clinical specimens. Comparison between health-care-worker collection and self-collection for pooled nasal and throat swabs and nasal swabs showed comparable diagnostic performance. No significant heterogeneity was observed in the analysis of pooled nasal and throat swabs and throat swabs, whereas moderate to substantial heterogeneity (I2 ≥30%) was observed in studies on saliva and nasal swabs.
Interpretation: Our review suggests that, compared with the gold standard of nasopharyngeal swabs, pooled nasal and throat swabs offered the best diagnostic performance of the alternative sampling approaches for diagnosis of SARS-CoV-2 infection in ambulatory care. Saliva and nasal swabs gave comparable and very good diagnostic performance and are clinically acceptable alternative specimen collection methods. Throat swabs gave a much lower sensitivity and positive predictive value and should not be recommended. Self-collection for pooled nasal and throat swabs and nasal swabs was not associated with any significant impairment of diagnostic accuracy. Our results also provide a useful reference framework for the proper interpretation of SARS-CoV-2 testing results using different clinical specimens.
Funding: Hong Kong Research Grants Council.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests BJC consults for Roche and Sanofi Pasteur. All other authors declare no competing interests.
Figures



Comment in
-
Beyond COVID-19-will self-sampling and testing become the norm?Lancet Infect Dis. 2021 Sep;21(9):1194-1195. doi: 10.1016/S1473-3099(21)00197-3. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857408 Free PMC article. No abstract available.
-
Is oropharyngeal sampling a reliable test to detect SARS-CoV-2? - Authors' reply.Lancet Infect Dis. 2021 Oct;21(10):1348-1349. doi: 10.1016/S1473-3099(21)00402-3. Epub 2021 Aug 4. Lancet Infect Dis. 2021. PMID: 34363770 Free PMC article. No abstract available.
-
Is oropharyngeal sampling a reliable test to detect SARS-CoV-2?Lancet Infect Dis. 2021 Oct;21(10):1348. doi: 10.1016/S1473-3099(21)00395-9. Epub 2021 Aug 4. Lancet Infect Dis. 2021. PMID: 34363773 Free PMC article. No abstract available.
-
Testing indicators to monitor the COVID-19 pandemic.Lancet Infect Dis. 2021 Oct;21(10):1344-1345. doi: 10.1016/S1473-3099(21)00461-8. Epub 2021 Aug 24. Lancet Infect Dis. 2021. PMID: 34450053 Free PMC article. No abstract available.
-
In suspected COVID-19, RT-PCR with nasal plus throat swabs has 97% sensitivity vs. nasopharyngeal swabs.Ann Intern Med. 2021 Sep;174(9):JC107. doi: 10.7326/ACPJ202109210-107. Epub 2021 Sep 7. Ann Intern Med. 2021. PMID: 34487439
References
-
- WHO Weekly epidemiological update on COVID-19. March 22, 2021. https://www.who.int/publications/m/item/weekly-operational-update-on-cov...
-
- WHO . World Health Organization; Geneva: 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

