Il4ra-independent vaginal eosinophil accumulation following helminth infection exacerbates epithelial ulcerative pathology of HSV-2 infection
- PMID: 33857419
- PMCID: PMC8062792
- DOI: 10.1016/j.chom.2021.02.004
Il4ra-independent vaginal eosinophil accumulation following helminth infection exacerbates epithelial ulcerative pathology of HSV-2 infection
Abstract
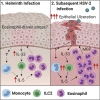
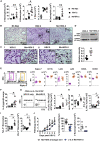
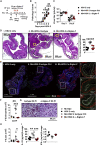
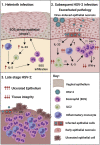
How helminths influence the pathogenesis of sexually transmitted viral infections is not comprehensively understood. Here, we show that an acute helminth infection (Nippostrongylus brasiliensis [Nb]) induced a type 2 immune profile in the female genital tract (FGT). This leads to heightened epithelial ulceration and pathology in subsequent herpes simplex virus (HSV)-2 infection. This was IL-5-dependent but IL-4 receptor alpha (Il4ra) independent, associated with increased FGT eosinophils, raised vaginal IL-33, and enhanced epithelial necrosis. Vaginal eosinophil accumulation was promoted by IL-33 induction following targeted vaginal epithelium damage from a papain challenge. Inhibition of IL-33 protected against Nb-exacerbated HSV-2 pathology. Eosinophil depletion reduced IL-33 release and HSV-2 ulceration in Nb-infected mice. These findings demonstrate that Nb-initiated FGT eosinophil recruitment promotes an eosinophil, IL-33, and IL-5 inflammatory circuit that enhances vaginal epithelial necrosis and pathology following HSV-2 infection. These findings identify a mechanistic framework as to how helminth infections can exacerbate viral-induced vaginal pathology.
Keywords: HSV-2; IL-33; IL-5; Nippostrongylus brasiliensis; eosinophils; epithelial ulceration; helminths; systemic immunity; vagina.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures




Comment in
-
Helminth virus co-infection: Implications for women's health.Cell Host Microbe. 2021 Apr 14;29(4):543-545. doi: 10.1016/j.chom.2021.03.014. Cell Host Microbe. 2021. PMID: 33857416
Similar articles
-
NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection.J Virol. 2005 Apr;79(7):4470-8. doi: 10.1128/JVI.79.7.4470-4478.2005. J Virol. 2005. PMID: 15767447 Free PMC article.
-
Novel Role for Interleukin-17 in Enhancing Type 1 Helper T Cell Immunity in the Female Genital Tract following Mucosal Herpes Simplex Virus 2 Vaccination.J Virol. 2017 Nov 14;91(23):e01234-17. doi: 10.1128/JVI.01234-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956763 Free PMC article.
-
IL-36γ induces a transient HSV-2 resistant environment that protects against genital disease and pathogenesis.Cytokine. 2018 Nov;111:63-71. doi: 10.1016/j.cyto.2018.07.034. Epub 2018 Aug 15. Cytokine. 2018. PMID: 30118914 Free PMC article.
-
Herpes simplex virus type 2 in sub-Saharan Africa and the potential impact of helminth immune modulation.Front Cell Infect Microbiol. 2024 Dec 4;14:1471411. doi: 10.3389/fcimb.2024.1471411. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39698320 Free PMC article. Review.
-
The role of dendritic cells in immune responses against vaginal infection by herpes simplex virus type 2.Microbes Infect. 2003 Nov;5(13):1221-30. doi: 10.1016/j.micinf.2003.09.006. Microbes Infect. 2003. PMID: 14623018 Review.
Cited by
-
Peripheral Eosinophil Count Is Associated With the Prognosis of Patients With Type B Aortic Dissection Undergoing Endovascular Aortic Repair: A Retrospective Cohort Study.J Am Heart Assoc. 2022 Dec 6;11(23):e027339. doi: 10.1161/JAHA.122.027339. Epub 2022 Nov 23. J Am Heart Assoc. 2022. PMID: 36416154 Free PMC article.
-
Induction of Siglec-FhiCD101hi eosinophils in the lungs following murine hookworm Nippostrongylus brasiliensis infection.Front Immunol. 2023 May 12;14:1170807. doi: 10.3389/fimmu.2023.1170807. eCollection 2023. Front Immunol. 2023. PMID: 37251384 Free PMC article.
-
Helminth-virus interactions: determinants of coinfection outcomes.Gut Microbes. 2021 Jan-Dec;13(1):1961202. doi: 10.1080/19490976.2021.1961202. Gut Microbes. 2021. PMID: 34428107 Free PMC article. Review.
-
Infection with soil-transmitted helminths and their impact on coinfections.Front Parasitol. 2023 May 24;2:1197956. doi: 10.3389/fpara.2023.1197956. eCollection 2023. Front Parasitol. 2023. PMID: 39816832 Free PMC article. Review.
-
Systemic Immune Modulation by Gastrointestinal Nematodes.Annu Rev Immunol. 2024 Jun;42(1):259-288. doi: 10.1146/annurev-immunol-090222-101331. Epub 2024 Jun 14. Annu Rev Immunol. 2024. PMID: 38277692 Free PMC article. Review.
References
-
- Blaho J.A., Morton E.R., Yedowitz J.C. Herpes simplex virus: propagation, quantification, and storage. Curr. Protoc. Microbiol. 2005:14E.1.1–14E.1.23. - PubMed
-
- Bonilla W.V., Fröhlich A., Senn K., Kallert S., Fernandez M., Johnson S., Kreutzfeldt M., Hegazy A.N., Schrick C., Fallon P.G. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335:984–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

