Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett's esophagus in a multisite case-control study
- PMID: 33857451
- PMCID: PMC8380660
- DOI: 10.1016/j.gie.2021.03.937
Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett's esophagus in a multisite case-control study
Abstract
Background and aims: We previously identified a 5 methylated DNA marker (MDM) panel for the detection of nonendoscopic Barrett's esophagus (BE). In this study, we aimed to recalibrate the performance of the 5 MDM panel using a simplified assay in a training cohort, validate the panel in an independent test cohort, and explore the accuracy of an MDM panel with only 3 markers.
Methods: Participants were recruited from 3 medical centers. The sponge on a string device (EsophaCap; CapNostics, Concord, NC, USA) was swallowed and withdrawn, followed by endoscopy, in BE cases and control subjects. A 5 MDM panel was blindly assayed using a simplified assay. Random forest modeling analysis was performed, in silico cross-validated in the training set, and then locked down, before test set analysis.
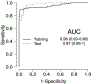
Results: The training set had 199 patients: 110 BE cases and 89 control subjects, and the test set had 89 patients: 60 BE cases and 29 control subjects. Sensitivity of the 5 MDM panel for BE diagnosis was 93% at 90% specificity in the training set and 93% at 93% specificity in the test set. Areas under the receiver operating characteristic curves were .96 and .97 in the training and test sets, respectively. Model accuracy was not influenced by age, sex, or smoking history. Multiple 3 MDM panels achieved similar accuracy.
Conclusions: A 5 MDM panel for BE is highly accurate in training and test sets in a blinded multisite case-control analysis using a simplified assay. This panel may be reduced to only 3 MDMs in the future. (Clinical trial registration number: NCT02560623.).
Copyright © 2021 American Society for Gastrointestinal Endoscopy. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Screening for Barrett's esophagus: Ready for prime time or still hard to swallow?Gastrointest Endosc. 2021 Sep;94(3):506-508. doi: 10.1016/j.gie.2021.06.004. Epub 2021 Jul 16. Gastrointest Endosc. 2021. PMID: 34275609 No abstract available.
References
-
- Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol 2009;104:1356–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

