Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers
- PMID: 33857476
- PMCID: PMC8056941
- DOI: 10.1016/j.cca.2021.04.006
Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers
Abstract
Background: Vaccine-induced population immunity is a key global strategy to control coronavirus disease 2019 (COVID-19). The rapid implementation and availability of several COVID-19 vaccines is now a global health-care priority but more information about humoral responses to single- and double-dose vaccine is needed.
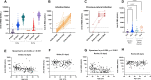
Methods: 163 health care workers (HCW) of the Padua University Hospitals, who underwent a complete vaccination campaign with BNT162b2 vaccine were asked to collect serum samples at 12 (t12) and 28 (t28) days after the first inoculum to allow the measurement of SARS-CoV-2 Antibodies (Ab) using chemiluminescent assays against the spike (S) protein and the Receptor Binding Domain (RBD) of the virus, respectively.
Results: Significant differences were found at t12 for infection-naïve and subjects with previous-natural infection who present higher values of specific antibodies, while no significant differences have been found between t12 and t28. No statistically significant difference was found between male and female, while lower Ab levels have been observed in subjects older than 60 years at t12 but not at t28.
Conclusions: Our study confirms observed differences in vaccine responses between infection-naïve and subjects with previous natural infection at t12 but not for a longer time. The influence of sex and age deserves further studies, even if the relationship with age seems particularly significant.
Keywords: Antibody; BNT162b2; COVID-19; Immunoassays; Immunological response; SARS-CoV-2 vaccine; Serology.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. - PMC - PubMed
-
- Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., Verheul M.K., Aley P.K., Angus B., Bellamy D., Berrie E., Bibi S., Bittaye M., Carroll M.W., Cavell B., Clutterbuck E.A., Edwards N., Flaxman A., Fuskova M., Gorringe A., Hallis B., Kerridge S., Lawrie A.M., Linder A., Liu X., Madhavan M., Makinson R., Mellors J., Minassian A., Moore M., Mujadidi Y., Plested E., Poulton I., Ramasamy M.N., Robinson H., Rollier C.S., Song R., Snape M.D., Tarrant R., Taylor S., Thomas K.M., Voysey M., Watson M.E.E., Wright D., Douglas A.D., Green C.M., Hill A.V.S., Lambe T., Gilbert S., Pollard A.J. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. - PubMed
-
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O’Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. - PMC - PubMed
-
- European center for disease prevention and control (ECDC), Overview of COVID-19 vaccination strategies and vaccine deployment plans in the EU / EEA and the UK Key findings, (2020) 1–22.
-
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

