Altered excitatory transmission in striatal neurons after chronic ethanol consumption in selectively bred crossed high alcohol-preferring mice
- PMID: 33857521
- PMCID: PMC8293703
- DOI: 10.1016/j.neuropharm.2021.108564
Altered excitatory transmission in striatal neurons after chronic ethanol consumption in selectively bred crossed high alcohol-preferring mice
Abstract
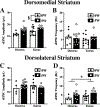
Genetic predisposition to heavy drinking is a risk factor for alcohol misuse. We used selectively bred crossed high alcohol-preferring (cHAP) mice to study sex differences in alcohol drinking and its effect on glutamatergic activity in dorsolateral (DLS) and dorsomedial (DMS) striatum. We performed whole-cell patch-clamp recording in neurons from male and female cHAP mice with 5-week alcohol drinking history and alcohol-naïve controls. In DMS, alcohol-naïve males' neurons displayed lower cell capacitance and higher membrane resistance than females' neurons, both effects reversed by drinking. Conversely, in DLS neurons, drinking history increased capacitance only in males and changed membrane resistance only in females. Altered biophysical membrane properties were accompanied by disrupted glutamatergic transmission. Drinking history increased spontaneous excitatory postsynaptic current (sEPSC) amplitude in DMS and frequency in DLS female neurons, compared to alcohol-naïve females, without effect in males. Acute ethanol differentially impacted DMS and DLS neurons by sex and drinking history. In DMS, acute alcohol significantly increased sEPSC frequency only in neurons from alcohol-naïve females, an effect that disappeared after drinking history. In DLS, acute alcohol had opposing effects in males and females based on drinking history. Estrous cycle also impacted DMS and DLS neurons differently: sEPSC amplitudes were higher in DMS cells from drinking history than alcohol-naïve females, whereas estrous cycle, not drinking history, modified DLS firing rate. Our data show sex differences in cHAP ethanol consumption and neurophysiology, suggesting differential dysregulation of glutamatergic drive onto DMS and DLS after chronic ethanol consumption.
Keywords: Alcohol misuse; Dorsal striatum; Estrous cycle; Genetically selected lines; Glutamate.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures
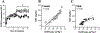

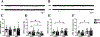


References
-
- Agrawal A, Lynskey MT, 2008. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction 103, 1069–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

