Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines
- PMID: 33857566
- PMCID: PMC8056854
- DOI: 10.1016/j.jaci.2021.04.002
Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines
Abstract
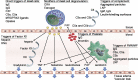
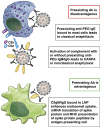
Anaphylaxis to vaccines is historically a rare event. The coronavirus disease 2019 pandemic drove the need for rapid vaccine production applying a novel antigen delivery system: messenger RNA vaccines packaged in lipid nanoparticles. Unexpectedly, public vaccine administration led to a small number of severe allergic reactions, with resultant substantial public concern, especially within atopic individuals. We reviewed the constituents of the messenger RNA lipid nanoparticle vaccine and considered several contributors to these reactions: (1) contact system activation by nucleic acid, (2) complement recognition of the vaccine-activating allergic effector cells, (3) preexisting antibody recognition of polyethylene glycol, a lipid nanoparticle surface hydrophilic polymer, and (4) direct mast cell activation, coupled with potential genetic or environmental predispositions to hypersensitivity. Unfortunately, measurement of anti-polyethylene glycol antibodies in vitro is not clinically available, and the predictive value of skin testing to polyethylene glycol components as a coronavirus disease 2019 messenger RNA vaccine-specific anaphylaxis marker is unknown. Even less is known regarding the applicability of vaccine use for testing (in vitro/vivo) to ascertain pathogenesis or predict reactivity risk. Expedient and thorough research-based evaluation of patients who have suffered anaphylactic vaccine reactions and prospective clinical trials in putative at-risk individuals are needed to address these concerns during a public health crisis.
Keywords: COVID-19 vaccine; PEGylated liposome; allergy; anaphylaxis; lipid nanoparticle; mRNA vaccine; mast cells; polyethylene glycol.
Copyright © 2021 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

