Investigation of a Prevascularized Bone Graft for Large Defects in the Ovine Tibia
- PMID: 33858216
- PMCID: PMC8742287
- DOI: 10.1089/ten.TEA.2020.0347
Investigation of a Prevascularized Bone Graft for Large Defects in the Ovine Tibia
Abstract
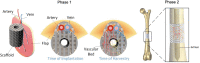
In vivo bioreactors are a promising approach for engineering vascularized autologous bone grafts to repair large bone defects. In this pilot parametric study, we first developed a three-dimensional (3D) printed scaffold uniquely designed to accommodate inclusion of a vascular bundle and facilitate growth factor delivery for accelerated vascular invasion and ectopic bone formation. Second, we established a new sheep deep circumflex iliac artery (DCIA) model as an in vivo bioreactor for engineering a vascularized bone graft and evaluated the effect of implantation duration on ectopic bone formation. Third, after 8 weeks of implantation around the DCIA, we transplanted the prevascularized bone graft to a 5 cm segmental bone defect in the sheep tibia, using the custom 3D printed bone morphogenic protein 2 (BMP-2) loaded scaffold without prior in vivo bioreactor maturation as a control. Analysis by micro-computed tomography and histomorphometry found ectopic bone formation in BMP-2 loaded scaffolds implanted for 8 and 12 weeks in the iliac pouch, with greater bone formation occurring after 12 weeks. Grafts transplanted to the tibial defect supported bone growth, mainly on the periphery of the graft, but greater bone growth and less soft tissue invasion was observed in the avascular BMP-2 loaded scaffold implanted directly into the tibia without prior in vivo maturation. Histopathological evaluation noted considerably greater vascularity in the bone grafts that underwent in vivo maturation with an inserted vascular bundle compared with the avascular BMP-2 loaded graft. Our findings indicate that the use of an initial DCIA in vivo bioreactor maturation step is a promising approach to developing vascularized autologous bone grafts, although scaffolds with greater osteoinductivity should be further studied. Impact statement This translational pilot study aims at combining a tissue engineering scaffold strategy, in vivo prevascularization, and a modified transplantation technique to accelerate large segmental bone defect repair. First, we three-dimensional (3D) printed a 5 cm scaffold with a unique design to facilitate vascular bundle inclusion and osteoinductive growth factor delivery. Second, we established a new sheep deep circumflex iliac artery model as an in vivo bioreactor for prevascularizing the novel 3D printed osteoinductive scaffold. Subsequently, we transplanted the prevascularized bone graft to a clinically relevant 5 cm segmental bone defect in the sheep tibia for bone regeneration.
Keywords: 3D printed scaffolds; in vivo bioreactor; large bone defect; prevascularization; sheep model.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors.Acta Biomater. 2016 Nov;45:72-84. doi: 10.1016/j.actbio.2016.09.013. Epub 2016 Sep 12. Acta Biomater. 2016. PMID: 27633319
-
Combining a Vascular Bundle and 3D Printed Scaffold with BMP-2 Improves Bone Repair and Angiogenesis.Tissue Eng Part A. 2021 Dec;27(23-24):1517-1525. doi: 10.1089/ten.TEA.2021.0049. Epub 2021 Jun 18. Tissue Eng Part A. 2021. PMID: 33906392 Free PMC article.
-
Osteoinductive 3D printed scaffold healed 5 cm segmental bone defects in the ovine metatarsus.Sci Rep. 2021 Mar 23;11(1):6704. doi: 10.1038/s41598-021-86210-5. Sci Rep. 2021. PMID: 33758338 Free PMC article.
-
[Research progress in three-dimensional-printed bone scaffolds combined with vascularized tissue flaps for segmental bone defect reconstruction].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025 May 15;39(5):639-646. doi: 10.7507/1002-1892.202503081. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025. PMID: 40368869 Free PMC article. Review. Chinese.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
Cited by
-
Recent Advances on Cell-Based Co-Culture Strategies for Prevascularization in Tissue Engineering.Front Bioeng Biotechnol. 2021 Nov 25;9:745314. doi: 10.3389/fbioe.2021.745314. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34900955 Free PMC article. Review.
-
An osteoinductive and biodegradable intramedullary implant accelerates bone healing and mitigates complications of bone transport in male rats.Nat Commun. 2023 Jul 24;14(1):4455. doi: 10.1038/s41467-023-40149-5. Nat Commun. 2023. PMID: 37488113 Free PMC article.
-
Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones.Biomedicines. 2023 Jan 24;11(2):325. doi: 10.3390/biomedicines11020325. Biomedicines. 2023. PMID: 36830862 Free PMC article. Review.
-
Probing the role of methyl methacrylate release from spacer materials in induced membrane bone healing.J Orthop Res. 2022 May;40(5):1065-1074. doi: 10.1002/jor.25147. Epub 2021 Aug 14. J Orthop Res. 2022. PMID: 34314063 Free PMC article.
-
In vivo prevascularization strategy enhances neovascularization of β-tricalcium phosphate scaffolds in bone regeneration.J Orthop Translat. 2022 Oct 15;37:143-151. doi: 10.1016/j.jot.2022.09.001. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36313532 Free PMC article.
References
-
- Holzer, G., Einhorn, T.A., and Majeska, R.J.. Estrogen regulation of growth and alkaline phosphatase expression by cultured human bone marrow stromal cells. J Orthop Res 20, 281, 2002. - PubMed
-
- Larsson, S., and Bauer, T.W.. Use of injectable calcium phosphate cement for fracture fixation: a review. Clin Orthop Relat Res 395, 23, 2002. - PubMed
-
- Conway, J.D. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am 41, 75, 2010; table of contents. - PubMed
-
- Hernandez-Fernandez, A., Vélez, R., Soldado, F., Saenz-Ríos, J.C., Barber, I., and Aguirre-Canyadell, M.. Effect of administration of platelet-rich plasma in early phases of distraction osteogenesis: an experimental study in an ovine femur model. Injury 44, 901, 2013. - PubMed
-
- Yang, J.-H., Kim, H.-J., Kim, S.-E., et al. . The effect of bone morphogenic protein-2-coated tri-calcium phosphate/hydroxyapatite on new bone formation in a rat model of femoral distraction osteogenesis. Cytotherapy 14, 315, 2012. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

