Colorectal cancer screening using a stool DNA-based SDC2 methylation test: a multicenter, prospective trial
- PMID: 33858326
- PMCID: PMC8050895
- DOI: 10.1186/s12876-021-01759-9
Colorectal cancer screening using a stool DNA-based SDC2 methylation test: a multicenter, prospective trial
Abstract
Background: Prevention and early detection of colorectal cancer (CRC) is a global priority, with many countries conducting population-based CRC screening programs. Although colonoscopy is the most accurate diagnostic method for early CRC detection, adherence remains low because of its invasiveness and the need for extensive bowel preparation. Non-invasive fecal occult blood tests or fecal immunochemical tests are available; however, their sensitivity is relatively low. Syndecan-2 (SDC2) is a stool-based DNA methylation marker used for early detection of CRC. Using the EarlyTect™-Colon Cancer test, the sensitivity and specificity of SDC2 methylation in stool DNA for detecting CRC were previously demonstrated to be greater than 90%. Therefore, a larger trial to validate its use for CRC screening in asymptomatic populations is now required.
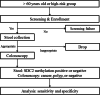
Methods: All participants will collect their stool (at least 20 g) before undergoing screening colonoscopy. The samples will be sent to a central laboratory for analysis. Stool DNA will be isolated using a GT Stool DNA Extraction kit, according to the manufacturer's protocol. Before performing the methylation test, stool DNA (2 µg per reaction) will be treated with bisulfite, according to manufacturer's instructions. SDC2 and COL2A1 control reactions will be performed in a single tube. The SDC2 methylation test will be performed using an AB 7500 Fast Real-time PCR system. CT values will be calculated using the 7500 software accompanying the instrument. Results from the EarlyTect™-Colon Cancer test will be compared against those obtained from colonoscopy and any corresponding diagnostic histopathology from clinically significant biopsied or subsequently excised lesions. Based on these results, participants will be divided into three groups: CRC, polyp, and negative. The following clinical data will be recorded for the participants: sex, age, colonoscopy results, and clinical stage (for CRC cases).
Discussion: This trial investigates the clinical performance of a device that allows quantitative detection of a single DNA marker, SDC2 methylation, in human stool DNA in asymptomatic populations. The results of this trial are expected to be beneficial for CRC screening and may help make colonoscopy a selective procedure used only in populations with a high risk of CRC.
Trial registration: This trial (NCT04304131) was registered at ClinicalTrials.gov on March 11, 2020 and is available at https://clinicaltrials.gov/ct2/show/NCT04304131?cond=NCT04304131&draw=2&rank=1 .
Keywords: Biomarker; Colorectal cancer; DNA methylation; Early detection; Syndecan-2.
Conflict of interest statement
The authors declare that they have no competing interests. However, EarlyTect™-Colon Cancer tests will be provided by Genomictree, Daejeon, Korea.
Figures
References
-
- Dashwood RH. Early detection and prevention of colorectal cancer (review) Oncol Rep. 1999;6(2):277–281. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous