A prospective multicenter assessor-blinded randomized controlled study to compare the efficacy of short versus long protocols of electroconvulsive therapy as an augmentation strategy to clozapine in patients with ultra-resistant schizophrenia (SURECT study)
- PMID: 33858488
- PMCID: PMC8048266
- DOI: 10.1186/s13063-021-05227-3
A prospective multicenter assessor-blinded randomized controlled study to compare the efficacy of short versus long protocols of electroconvulsive therapy as an augmentation strategy to clozapine in patients with ultra-resistant schizophrenia (SURECT study)
Abstract
Background: Although clozapine is the most effective antipsychotic drug for treatment-resistant schizophrenia, it leads to a poor or partial response in 40 to 70% of patients. Augmentation of clozapine with electroconvulsive therapy (ECT) is a highly effective and relatively safe treatment for these clozapine-resistant patients. However, parameters are not yet well specified, such as the optimal number of sessions, their frequency, and the relevance of maintenance ECT. Our objective is to compare the efficacy and tolerance between two protocols of combined ECT and clozapine treatment in patients with ultra-resistant schizophrenia (URS): a 6-month protocol (short protocol with 20 ECT sessions) and a 12-month protocol (long protocol with 40 ECT sessions).
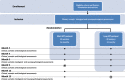
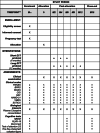
Methods: Sixty-four patients with schizophrenia with persistent psychotic symptoms despite clozapine treatment will be enrolled in a prospective multicentric assessor-blinded randomized controlled trial. Patients will be randomly assigned to the short or the long protocol. The main outcome is the response rate assessed by the Positive and Negative Symptoms Scale (PANSS) 3 months after the end of the treatment in patients following the long protocol compared to those following the short protocol. The response was defined as a 30% reduction on the PANSS baseline. Clinical assessments (PANSS, BPRS, HAMD-21, YMRS, CGI, GAF, Modified Overt Aggression Scale (MOAS), and Subjective Scale to Investigate Cognition in Schizophrenia (SSTICS)) and plasma clozapine concentration will be performed at baseline and at 2, 4, 6, 9, 12, and 15 months. Neuropsychological measures (MMSE, RL/RI-16, Doors test, D2 Test of Attention, Copy of the Rey-Osterrieth complex figure) will be performed at baseline and at 6 and 15 months.
Discussion: The aims of this research are to optimize protocols of combined ECT with clozapine in patients with URS and to offer specific recommendations for these patients' care.
Trial registration: ClinicalTrials.gov NCT03542903 . Registered on May 31, 2018. Id RCB: 2017-A02657-46.
Keywords: Augmentation strategy; Clozapine; Electroconvulsive therapy; Randomized controlled trial; Ultra-resistant schizophrenia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Randomized, double-blind, sham-controlled trial to evaluate the efficacy and tolerability of electroconvulsive therapy in patients with clozapine-resistant schizophrenia.Schizophr Res. 2024 Jun;268:252-260. doi: 10.1016/j.schres.2023.11.009. Epub 2023 Dec 26. Schizophr Res. 2024. PMID: 38151432 Clinical Trial.
-
Comparison of Acute Followed by Maintenance ECT vs Clozapine on Psychopathology and Regional Cerebral Blood Flow in Treatment-Resistant Schizophrenia: A Randomized Controlled Trial.Schizophr Bull. 2022 Jun 21;48(4):814-825. doi: 10.1093/schbul/sbac027. Schizophr Bull. 2022. PMID: 35556138 Free PMC article. Clinical Trial.
-
Electroconvulsive Therapy Versus Aripiprazole Addition to Clozapine in Patients with Clozapine-Resistant Symptoms (EMECLO): A Protocol of a Single-Blind, Multicenter, Randomized-Controlled Feasibility Trial.Pharmacopsychiatry. 2024 Nov;57(6):290-295. doi: 10.1055/a-2364-4357. Epub 2024 Aug 26. Pharmacopsychiatry. 2024. PMID: 39187245
-
Schizophrenia: when clozapine fails.Curr Opin Psychiatry. 2015 May;28(3):243-8. doi: 10.1097/YCO.0000000000000159. Curr Opin Psychiatry. 2015. PMID: 25768082 Review.
-
Is electroconvulsive therapy effective as augmentation in clozapine-resistant schizophrenia?Medwave. 2016 Oct 14;16(Suppl5):e6577. doi: 10.5867/medwave.2016.6577. Medwave. 2016. PMID: 27813505 Review. English, Spanish.
Cited by
-
High frequency repetitive transcranial magnetic stimulation for auditory verbal hallucinations in schizophrenia-spectrum disorders: a naturalistic study.Front Psychiatry. 2025 Jun 5;16:1551901. doi: 10.3389/fpsyt.2025.1551901. eCollection 2025. Front Psychiatry. 2025. PMID: 40539027 Free PMC article.
-
Clozapine: Why Is It So Uniquely Effective in the Treatment of a Range of Neuropsychiatric Disorders?Biomolecules. 2021 Jul 15;11(7):1030. doi: 10.3390/biom11071030. Biomolecules. 2021. PMID: 34356654 Free PMC article. Review.
-
Efficacy of Rivastigmine Augmentation on Positive and Negative Symptoms, General Psychopathology, and Quality of Life in Patients with Chronic Schizophrenia: A Randomized Controlled Trial.Psychopharmacol Bull. 2024 Apr 4;54(2):15-27. Psychopharmacol Bull. 2024. PMID: 38601834 Free PMC article. Clinical Trial.
-
Neuropsychological differences between treatment-resistant and treatment-responsive schizophrenia: a meta-analysis.Psychol Med. 2022 Jan;52(1):1-13. doi: 10.1017/S0033291721004128. Epub 2021 Nov 1. Psychol Med. 2022. PMID: 36415088 Free PMC article.
References
-
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ, the Wfsbp Task Force on Treatment Guidelines for Schizophrenia World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. doi: 10.3109/15622975.2012.696143. - DOI - PubMed
-
- Iasevoli F, Giordano S, Balletta R, Latte G, Formato MV, Prinzivalli E, de Berardis D, Tomasetti C, de Bartolomeis A. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;65:34–48. doi: 10.1016/j.pnpbp.2015.08.010. - DOI - PubMed
-
- Petrides G, Malur C, Braga RJ, Bailine SH, Schooler NR, Malhotra AK, Kane JM, Sanghani S, Goldberg TE, John M, Mendelowitz A. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am J Psychiatry. 2015;172(1):52–58. doi: 10.1176/appi.ajp.2014.13060787. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

