Stereotactic cisternal lavage in patients with aneurysmal subarachnoid hemorrhage with urokinase and nimodipine for the prevention of secondary brain injury (SPLASH): study protocol for a randomized controlled trial
- PMID: 33858493
- PMCID: PMC8048077
- DOI: 10.1186/s13063-021-05208-6
Stereotactic cisternal lavage in patients with aneurysmal subarachnoid hemorrhage with urokinase and nimodipine for the prevention of secondary brain injury (SPLASH): study protocol for a randomized controlled trial
Abstract
Background: Delayed cerebral infarction (DCI) is a major cause of death and poor neurological outcome in patients with aneurysmal subarachnoid hemorrhage (aSAH). Direct intrathecal therapies with fibrinolytic and spasmolytic drugs have appeared promising in clinical trials. However, access to the subarachnoid space for intrathecal drug administration is an unsolved problem so far, especially in patients with endovascular aneurysm securing. We investigate a therapy protocol based on stereotactic catheter ventriculocisternostomy (STX-VCS), a new approach to overcome this problem. The primary objective of this study is to assess whether cisternal lavage with urokinase, nimodipine, and Ringer's solution administered via a stereotactically implanted catheter into the basal cisterns (= investigational treatment (IT)) is safe and improves neurological outcome in patients with aSAH.
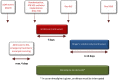
Methods: This is a randomized, controlled, parallel-group, open-label phase II trial. Fifty-four patients with severe aSAH (WFNS grade ≥ 3) will be enrolled at one academic tertiary care center in Southern Germany. Patients will be randomized at a ratio of 1:1 to receive either standard of care only or standard of care plus the IT. The primary endpoint is the proportion of subjects with a favorable outcome on the Modified Rankin Scale (defined as mRS 0-3) at 6 months after aSAH. Further clinical and surrogate outcome parameters are defined as secondary endpoints.
Discussion: New approaches for the prevention and therapy of secondary brain injury in patients with aSAH are urgently needed. We propose this RCT to assess the clinical safety and efficacy of a novel therapy protocol for intrathecal administration of urokinase, nimodipine, and Ringer's solution.
Trial registration: Deutsches Register Klinischer Studien (German Clinical Trials Register), DRKS00015645 . Registered on 8 May 2019.
Keywords: Aneurysmal subarachnoid hemorrhage (aSAH); Clinical trial; Delayed cerebral infarction (DCI); Intracisternal lavage; Intrathecal treatment; Nimodipine; Stereotactic ventriculocisternostomy (STX-VCS); Urokinase.
Conflict of interest statement
Dr. Roelz and Dr. Reinacher report a research grant from Else Kröner-Fresenius-Stiftung for the conduct of the study. Dr. Reinacher reports personal fees from Fraunhofer-Gesellschaft (Munich, Germany), Boston Scientific (Marlborough, MA, USA), Brainlab (Munich, Germany), and Inomed (Emmendingen, Germany) outside the submitted work. The other authors declare that they have no competing interests.
Figures
Similar articles
-
Impact of Stereotactic Ventriculocisternostomy on Delayed Cerebral Infarction and Outcome After Subarachnoid Hemorrhage.Stroke. 2020 Feb;51(2):431-439. doi: 10.1161/STROKEAHA.119.027424. Epub 2019 Dec 4. Stroke. 2020. PMID: 31795898
-
Stereotactic Catheter Ventriculocisternostomy for Clearance of Subarachnoid Hemorrhage in Patients with Coiled Aneurysms.Oper Neurosurg. 2018 Mar 1;14(3):231-235. doi: 10.1093/ons/opx129. Oper Neurosurg. 2018. PMID: 28582545
-
Clinical Trial Protocol: Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study Comparing EG-1962 to Standard of Care Oral Nimodipine in Adults with Aneurysmal Subarachnoid Hemorrhage [NEWTON-2 (Nimodipine Microparticles to Enhance Recovery While Reducing TOxicity After SubarachNoid Hemorrhage)].Neurocrit Care. 2019 Feb;30(1):88-97. doi: 10.1007/s12028-018-0575-z. Neurocrit Care. 2019. PMID: 30014184
-
Update on intrathecal management of cerebral vasospasm: a systematic review and meta-analysis.Neurosurg Focus. 2022 Mar;52(3):E10. doi: 10.3171/2021.12.FOCUS21629. Neurosurg Focus. 2022. PMID: 35231885
-
Intrathecal application of the nimodipine slow-release microparticle system eg-1962 for prevention of delayed cerebral ischemia and improvement of outcome after aneurysmal subarachnoid hemorrhage.Acta Neurochir Suppl. 2015;120:281-6. doi: 10.1007/978-3-319-04981-6_47. Acta Neurochir Suppl. 2015. PMID: 25366637 Review.
Cited by
-
Contemporary management of aneurysmal subarachnoid haemorrhage. An update for the intensivist.Intensive Care Med. 2024 May;50(5):646-664. doi: 10.1007/s00134-024-07387-7. Epub 2024 Apr 10. Intensive Care Med. 2024. PMID: 38598130 Free PMC article. Review.
-
Treatment factors to suppress delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage based on VASOGRADE: multicenter cohort study.Neurosurg Rev. 2024 Sep 7;47(1):564. doi: 10.1007/s10143-024-02795-1. Neurosurg Rev. 2024. PMID: 39242404
-
Thrombolysis for aneurysmal subarachnoid haemorrhage.Cochrane Database Syst Rev. 2025 Jan 17;1(1):CD013748. doi: 10.1002/14651858.CD013748.pub2. Cochrane Database Syst Rev. 2025. PMID: 39822092
-
Targeting Hemoglobin to Reduce Delayed Cerebral Ischemia After Subarachnoid Hemorrhage.Transl Stroke Res. 2022 Oct;13(5):725-735. doi: 10.1007/s12975-022-00995-9. Epub 2022 Feb 14. Transl Stroke Res. 2022. PMID: 35157256 Free PMC article. Review.
-
Prevention of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage-Summary of Existing Clinical Evidence.Transl Stroke Res. 2025 Feb;16(1):2-17. doi: 10.1007/s12975-024-01292-3. Epub 2024 Aug 30. Transl Stroke Res. 2025. PMID: 39212835 Review.
References
-
- Vergouwen MDI, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2011;31(7):1545–1553. doi: 10.1038/jcbfm.2011.56. - DOI - PMC - PubMed
-
- Jabbarli R, Reinhard M, Roelz R, Shah M, Niesen W-D, Kaier K, Taschner C, Weyerbrock A, Velthoven VV. Early identification of individuals at high risk for cerebral infarction after aneurysmal subarachnoid hemorrhage: the BEHAVIOR score. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35(10):1587–1592. doi: 10.1038/jcbfm.2015.81. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources