A randomized, controlled clinical trial of autologous stromal vascular fraction cells transplantation to promote mechanical stretch-induced skin regeneration
- PMID: 33858504
- PMCID: PMC8048343
- DOI: 10.1186/s13287-021-02318-5
A randomized, controlled clinical trial of autologous stromal vascular fraction cells transplantation to promote mechanical stretch-induced skin regeneration
Abstract
Background: The regeneration response of the skin to mechanical stretching in vivo has been explored in reconstructive surgery to repair large-scale deformities. The ability of the skin to regenerate limits the reconstructive outcome. Here, we propose an approach in which autologous stromal vascular fraction (SVF) cells and mechanical stretching are combined to overcome this limitation and promote skin regeneration.
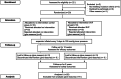
Methods: This randomized, blinded, placebo-controlled clinical trial screened 22 participants undergoing tissue expansion with exhausted regeneration. Twenty eligible participants received intradermal injections of the SVF or placebo treatments. Follow-ups were conducted at 4, 8, and 12 weeks to assess efficacy and at 2 years to assess safety. The primary endpoint was the expanded skin thickness at 12 weeks. The secondary endpoints included skin thickness at 4 and 8 weeks, the expansion index (EI), and the skin texture score at 12 weeks.
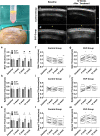
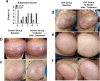
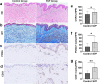
Results: The skin thickness of the SVF group was significantly higher than that of the control group at both 8 weeks (mean difference 0.78 [95% CI - 1.43 to - 0.11]; p = 0.018) and 12 weeks (0.65 [95% CI - 1.30 to - 0.01]; p = 0.046). In the SVF group, the increase in skin thickness was significant at 4 weeks (0.49 [95% CI - 0.80 to - 0.06]; p = 0.010) to 8 weeks (0.45 [95% CI - 0.92 to 0.02]; p = 0.026) and maintained after 12 weeks, whereas that in the control group was reduced after 8 weeks (0.42 [95% CI - 0.07 to 0.91]; p = 0.037). The SVF group showed greater EI increases than the control group (0.50 [95% CI - 0.00 to 0.99]; p = 0.047). The skin texture scores in the SVF group were greater than those in the control group at 12 weeks. Histologically, SVF-treated expanded skin showed more proliferating cells and blood vessels, and the extracellular matrix volume increased. No severe adverse events occurred.
Conclusions: Transplantation of SVF cells can expedite the potency of mechanical stretch-induced skin regeneration and provide clinical reconstruction with plentiful tissue.
Trial registration: This trial was registered with the Chinese Clinical Trial Registry, ChiCTR2000039317 (registered 23 October 2020-retrospectively registered).
Keywords: Mechanical stretch; Skin expansion; Skin regeneration; Stem cell; Stromal vascular fraction (SVF).
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

Similar articles
-
Isolation of the Stromal Vascular Fraction Using a New Protocol with All Clinical-Grade Drugs: From Basic Study to Clinical Application.Aesthetic Plast Surg. 2024 Nov;48(22):4702-4711. doi: 10.1007/s00266-024-04221-9. Epub 2024 Jul 10. Aesthetic Plast Surg. 2024. PMID: 38987318 Clinical Trial.
-
Enhancing Skin Regeneration During Expansion: A Multicenter Randomized Controlled Trial of Stromal Vascular Fraction and Fat Grafting.Plast Reconstr Surg. 2025 Aug 11. doi: 10.1097/PRS.0000000000012347. Online ahead of print. Plast Reconstr Surg. 2025. PMID: 40794208
-
Autologous Stem Cell Transplantation Promotes Mechanical Stretch Induced Skin Regeneration: A Randomized Phase I/II Clinical Trial.EBioMedicine. 2016 Nov;13:356-364. doi: 10.1016/j.ebiom.2016.09.031. Epub 2016 Oct 1. EBioMedicine. 2016. PMID: 27876353 Free PMC article. Clinical Trial.
-
Autologous adipose stem cell therapy for knee osteoarthritis: where are we now?Phys Sportsmed. 2020 Nov;48(4):392-399. doi: 10.1080/00913847.2020.1758001. Epub 2020 Apr 27. Phys Sportsmed. 2020. PMID: 32312142
-
Concise Review: Using Fat to Fight Disease: A Systematic Review of Nonhomologous Adipose-Derived Stromal/Stem Cell Therapies.Stem Cells. 2018 Sep;36(9):1311-1328. doi: 10.1002/stem.2847. Epub 2018 Jul 16. Stem Cells. 2018. PMID: 29761573
Cited by
-
Metformin Promotes Mechanical Stretch-Induced Skin Regeneration by Improving the Proliferative Activity of Skin-Derived Stem Cells.Front Med (Lausanne). 2022 May 24;9:813917. doi: 10.3389/fmed.2022.813917. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35685420 Free PMC article.
-
Efficacy and safety of epidermal cell regeneration for postoperative non-healing wounds: a randomized, partially blinded, parallel-controlled trial.Stem Cell Res Ther. 2025 Jun 3;16(1):284. doi: 10.1186/s13287-025-04425-z. Stem Cell Res Ther. 2025. PMID: 40462225 Free PMC article.
-
Isolation of the Stromal Vascular Fraction Using a New Protocol with All Clinical-Grade Drugs: From Basic Study to Clinical Application.Aesthetic Plast Surg. 2024 Nov;48(22):4702-4711. doi: 10.1007/s00266-024-04221-9. Epub 2024 Jul 10. Aesthetic Plast Surg. 2024. PMID: 38987318 Clinical Trial.
-
Therapeutic effect of adipose stromal vascular fraction spheroids for partial bladder outlet obstruction induced underactive bladder.Stem Cell Res Ther. 2022 Feb 9;13(1):68. doi: 10.1186/s13287-022-02739-w. Stem Cell Res Ther. 2022. PMID: 35139904 Free PMC article.
-
Engineered stromal vascular fraction for tissue regeneration.Front Pharmacol. 2025 Mar 13;16:1510508. doi: 10.3389/fphar.2025.1510508. eCollection 2025. Front Pharmacol. 2025. PMID: 40183080 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

