Arsenic trioxide synergistically promotes the antileukaemic activity of venetoclax by downregulating Mcl-1 in acute myeloid leukaemia cells
- PMID: 33858507
- PMCID: PMC8051086
- DOI: 10.1186/s40164-021-00221-6
Arsenic trioxide synergistically promotes the antileukaemic activity of venetoclax by downregulating Mcl-1 in acute myeloid leukaemia cells
Abstract
Background: The evasion of apoptosis through dysregulated Bcl-2 family members is a hallmark of leukaemia stem cells (LSCs) in acute myeloid leukaemia (AML). Therefore, targeting Bcl-2 with venetoclax has been suggested as an attractive strategy for inducing apoptosis in AML LSCs. However, the selective inhibition of Bcl-2 in AML often leads to upregulation of Mcl-1, another dominant anti-apoptotic Bcl-2 family protein conferring venetoclax resistance.
Methods: We assessed the combined effect of venetoclax and arsenic trioxide (ATO) on leukaemic cell viability, apoptosis, combination index, and cell cycle in the human LSC-like KG1 and KG1a cells. The synergistic effect of venetoclax and ATO on apoptosis was also examined in primary CD34+ and CD34+CD38- LSCs from the bone marrow (BM) of AML patients, and compared with those from healthy donors.
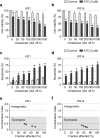
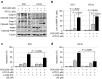
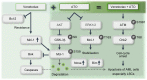
Results: Venetoclax efficiently impaired cell viability and dose-dependently promoted apoptosis when combined with ATO; their synergism was aptly represented by the combination index. The combination of venetoclax and ATO impaired cell cycle progression by restricting cells within the sub-G1 phase and facilitating caspase-dependent apoptotic cell death associated with the loss of mitochondrial membrane potential, while sparing healthy BM haematopoietic stem cells. Mechanistically, ATO mitigated venetoclax-induced upregulation of Mcl-1 by the inhibition of AKT and ERK, along with activation of GSK-3β. This led to the Mcl-1 destabilisation, triggering Noxa and Bim to facilitate apoptosis and the consequent activation of the apoptosis executioner protein Bak. Moreover, the combination promoted phosphorylation of ATM, Chk2, p38, and H2AX, indicating an active DNA damage response.
Conclusions: Our findings demonstrate the synergistic, preferential antileukaemic effects of venetoclax and ATO on LSCs, providing a rationale for preclinical and clinical trials by combining these agents already being used in clinical practice to treat acute leukaemia.
Keywords: Acute myeloid leukaemia; Apoptosis; Arsenic trioxide; Venetoclax.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





Similar articles
-
Arsenic Trioxide and Venetoclax Synergize against AML Progenitors by ROS Induction and Inhibition of Nrf2 Activation.Int J Mol Sci. 2022 Jun 12;23(12):6568. doi: 10.3390/ijms23126568. Int J Mol Sci. 2022. PMID: 35743010 Free PMC article.
-
Synergistic killing effects of homoharringtonine and arsenic trioxide on acute myeloid leukemia stem cells and the underlying mechanisms.J Exp Clin Cancer Res. 2019 Jul 15;38(1):308. doi: 10.1186/s13046-019-1295-8. J Exp Clin Cancer Res. 2019. PMID: 31307525 Free PMC article.
-
Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models.J Hematol Oncol. 2016 Jun 28;9(1):50. doi: 10.1186/s13045-016-0280-3. J Hematol Oncol. 2016. PMID: 27353420 Free PMC article.
-
Shutting Down Acute Myeloid Leukemia and Myelodysplastic Syndrome with BCL-2 Family Protein Inhibition.Curr Hematol Malig Rep. 2018 Aug;13(4):256-264. doi: 10.1007/s11899-018-0464-8. Curr Hematol Malig Rep. 2018. PMID: 29982865 Review.
-
Targeting Apoptotic Pathways in Acute Myeloid Leukaemia.Cancers (Basel). 2019 Oct 26;11(11):1660. doi: 10.3390/cancers11111660. Cancers (Basel). 2019. PMID: 31717784 Free PMC article. Review.
Cited by
-
Venetoclax in Acute Myeloid Leukemia: Molecular Basis, Evidences for Preclinical and Clinical Efficacy and Strategies to Target Resistance.Cancers (Basel). 2021 Nov 9;13(22):5608. doi: 10.3390/cancers13225608. Cancers (Basel). 2021. PMID: 34830763 Free PMC article. Review.
-
Arsenic Trioxide and Venetoclax Synergize against AML Progenitors by ROS Induction and Inhibition of Nrf2 Activation.Int J Mol Sci. 2022 Jun 12;23(12):6568. doi: 10.3390/ijms23126568. Int J Mol Sci. 2022. PMID: 35743010 Free PMC article.
-
Metabolic plasticity in blast crisis-chronic myeloid leukaemia cells under hypoxia reduces the cytotoxic potency of drugs targeting mitochondria.Discov Oncol. 2022 Jul 8;13(1):60. doi: 10.1007/s12672-022-00524-y. Discov Oncol. 2022. PMID: 35802257 Free PMC article.
-
Progress in understanding the mechanisms of resistance to BCL-2 inhibitors.Exp Hematol Oncol. 2022 May 21;11(1):31. doi: 10.1186/s40164-022-00283-0. Exp Hematol Oncol. 2022. PMID: 35598030 Free PMC article. Review.
-
Feasibility and Safety of Targeting Mitochondria Function and Metabolism in Acute Myeloid Leukemia.Curr Pharmacol Rep. 2024 Dec;10(6):388-404. doi: 10.1007/s40495-024-00378-8. Epub 2024 Oct 4. Curr Pharmacol Rep. 2024. PMID: 40756330 Free PMC article.
References
-
- Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13(1):72. doi: 10.1186/s13045-020-00908-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

