Clinical effect and antiviral mechanism of T-705 in treating severe fever with thrombocytopenia syndrome
- PMID: 33859168
- PMCID: PMC8050330
- DOI: 10.1038/s41392-021-00541-3
Clinical effect and antiviral mechanism of T-705 in treating severe fever with thrombocytopenia syndrome
Abstract
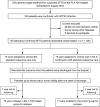
Severe fever with thrombocytopenia syndrome (SFTS) virus (SFTSV) is an emerging tick-borne virus with high fatality and an expanding endemic. Currently, effective anti-SFTSV intervention remains unavailable. Favipiravir (T-705) was recently reported to show in vitro and in animal model antiviral efficacy against SFTSV. Here, we conducted a single-blind, randomized controlled trial to assess the efficacy and safety of T-705 in treating SFTS (Chinese Clinical Trial Registry website, number ChiCTR1900023350). From May to August 2018, laboratory-confirmed SFTS patients were recruited from a designated hospital and randomly assigned to receive oral T-705 in combination with supportive care or supportive care only. Fatal outcome occurred in 9.5% (7/74) of T-705 treated patients and 18.3% (13/71) of controls (odds ratio, 0.466, 95% CI, 0.174-1.247). Cox regression showed a significant reduction in case fatality rate (CFR) with an adjusted hazard ratio of 0.366 (95% CI, 0.142-0.944). Among the low-viral load subgroup (RT-PCR cycle threshold ≥26), T-705 treatment significantly reduced CFR from 11.5 to 1.6% (P = 0.029), while no between-arm difference was observed in the high-viral load subgroup (RT-PCR cycle threshold <26). The T-705-treated group showed shorter viral clearance, lower incidence of hemorrhagic signs, and faster recovery of laboratory abnormities compared with the controls. The in vitro and animal experiments demonstrated that the antiviral efficacies of T-705 were proportionally induced by SFTSV mutation rates, particularly from two transition mutation types. The mutation analyses on T-705-treated serum samples disclosed a partially consistent mutagenesis pattern as those of the in vitro or animal experiments in reducing the SFTSV viral loads, further supporting the anti-SFTSV effect of T-705, especially for the low-viral loads.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

