Reconstitution of contractile actomyosin rings in vesicles
- PMID: 33859190
- PMCID: PMC8050101
- DOI: 10.1038/s41467-021-22422-7
Reconstitution of contractile actomyosin rings in vesicles
Erratum in
-
Author Correction: Reconstitution of contractile actomyosin rings in vesicles.Nat Commun. 2022 Mar 10;13(1):1383. doi: 10.1038/s41467-022-29063-4. Nat Commun. 2022. PMID: 35273165 Free PMC article. No abstract available.
Abstract
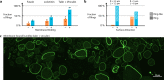
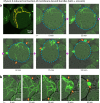
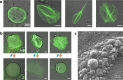
One of the grand challenges of bottom-up synthetic biology is the development of minimal machineries for cell division. The mechanical transformation of large-scale compartments, such as Giant Unilamellar Vesicles (GUVs), requires the geometry-specific coordination of active elements, several orders of magnitude larger than the molecular scale. Of all cytoskeletal structures, large-scale actomyosin rings appear to be the most promising cellular elements to accomplish this task. Here, we have adopted advanced encapsulation methods to study bundled actin filaments in GUVs and compare our results with theoretical modeling. By changing few key parameters, actin polymerization can be differentiated to resemble various types of networks in living cells. Importantly, we find membrane binding to be crucial for the robust condensation into a single actin ring in spherical vesicles, as predicted by theoretical considerations. Upon force generation by ATP-driven myosin motors, these ring-like actin structures contract and locally constrict the vesicle, forming furrow-like deformations. On the other hand, cortex-like actin networks are shown to induce and stabilize deformations from spherical shapes.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rottner K, Faix J, Bogdan S, Linder S, Kerkhoff E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017;130:3427–3435. - PubMed
-
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014;94:235–263. - PubMed
-
- Schwayer C, Sikora M, Slovakova J, Kardos R, Heisenberg CP. Actin rings of power. Dev. Cell. 2016;37:493–506. - PubMed
-
- Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 2008;37:65–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

