Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials
- PMID: 33859192
- PMCID: PMC8050319
- DOI: 10.1038/s41467-021-22446-z
Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials
Erratum in
-
Author Correction: Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials.Nat Commun. 2021 May 14;12(1):3001. doi: 10.1038/s41467-021-23559-1. Nat Commun. 2021. PMID: 33990619 Free PMC article. No abstract available.
-
Author Correction: Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials.Nat Commun. 2024 Feb 5;15(1):1075. doi: 10.1038/s41467-024-45360-6. Nat Commun. 2024. PMID: 38316844 Free PMC article. No abstract available.
Abstract
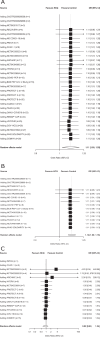
Substantial COVID-19 research investment has been allocated to randomized clinical trials (RCTs) on hydroxychloroquine/chloroquine, which currently face recruitment challenges or early discontinuation. We aim to estimate the effects of hydroxychloroquine and chloroquine on survival in COVID-19 from all currently available RCT evidence, published and unpublished. We present a rapid meta-analysis of ongoing, completed, or discontinued RCTs on hydroxychloroquine or chloroquine treatment for any COVID-19 patients (protocol: https://osf.io/QESV4/ ). We systematically identified unpublished RCTs (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, Cochrane COVID-registry up to June 11, 2020), and published RCTs (PubMed, medRxiv and bioRxiv up to October 16, 2020). All-cause mortality has been extracted (publications/preprints) or requested from investigators and combined in random-effects meta-analyses, calculating odds ratios (ORs) with 95% confidence intervals (CIs), separately for hydroxychloroquine and chloroquine. Prespecified subgroup analyses include patient setting, diagnostic confirmation, control type, and publication status. Sixty-three trials were potentially eligible. We included 14 unpublished trials (1308 patients) and 14 publications/preprints (9011 patients). Results for hydroxychloroquine are dominated by RECOVERY and WHO SOLIDARITY, two highly pragmatic trials, which employed relatively high doses and included 4716 and 1853 patients, respectively (67% of the total sample size). The combined OR on all-cause mortality for hydroxychloroquine is 1.11 (95% CI: 1.02, 1.20; I² = 0%; 26 trials; 10,012 patients) and for chloroquine 1.77 (95%CI: 0.15, 21.13, I² = 0%; 4 trials; 307 patients). We identified no subgroup effects. We found that treatment with hydroxychloroquine is associated with increased mortality in COVID-19 patients, and there is no benefit of chloroquine. Findings have unclear generalizability to outpatients, children, pregnant women, and people with comorbidities.
Conflict of interest statement
B.S.A. and R.K.A. are the primary investigators of the Prevention and Treatment of COVID19 with Hydroxychloroquine (PATCH) trial, funded by a philanthropic gift. R.K.A reports being founder with equity of Pinpoint Therapeutics and Immunacell, and personal fees from Sprint Biosciences and Deciphera, outside the submitted work. D.C.A. reports personal fees from Ferring Pharmaceuticals, Inc., Bristol-Myers Squibb, and Bayer AG, other from Alung Technologies, Inc., outside the submitted work; in addition, D.C.A. has pending patents for Selepressin—compounds, compositions, and methods for treating sepsis to Ferring, B.V., and Proteomic biomarkers of sepsis in elderly patients pending to University of Pittsburgh. Y.M.A. reports that he is the principal investigator on a clinical trial of lopinavir–ritonavir and interferon for Middle East respiratory syndrome (MERS) and that he was a nonpaid consultant on therapeutics for MERS-coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is a coinvestigator on the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP), a board member of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC), and the Lead-Co Chair of the Think20 (T20) Taskforce for COVID-19. Brigham and Women’s Hospital, PRA Health Science, and Cliniques universitaires Saint-Luc received funds from Sanofi. T.B. reports grants from Pfizer, Novo Nordisk Foundation, Simonsen Foundation, Lundbeck Foundation, and Kai Hansen Foundation; grants and personal fees from GSK, Pfizer, Boehringer Ingelheim, and Gilead; and personal fees from MSD, all outside the submitted work. Y.Z.C., L.N.C., B.I., and L.P. are employees of Sanofi. The COV-HCQ and COMIHY trials were supported by the German Federal Ministry of Education and Research (EudraCT number 2020-001224-33) and the German Federal Ministry of Health (EudraCT number 2020-001512-26). L.D. reports grants from EU FP7-HEALTH-2013-INNOVATION-1, grant number 602525, grants from H2020 RECOVER, grant agreement no. 101003589, during the conduct of the study; and is a member of the COVID-19 guideline committee SCCM/ESICM/SSC, member of the ESICM COVID-19 taskforce, and chair of the Dutch intensivists (NVIC) taskforce infectious threats. V.D. reports nonfinancial support from MSD France and from Sanofi Aventis France, outside the submitted work. A.E. is an employee of Ividata Life Sciences and works as an external contractor for Sanofi. A.C.G. received grant funding from an NIHR Research Professorship (RP-2015-06-18), support from the NIHR Imperial Biomedical Research Centre, and consulting fees paid to his institution from GlaxoSmithKline and Bristol-Myers Squibb. T.H. reports grants from the Health Research Council of New Zealand, during the conduct of the study. A.I.M.H. reports grants from ZonMw, Netherlands organisation for Health Research and Development, during the conduct of the study. HYDRA trial was an investigator-initiated study supported by Sanofi, CONACYT (National Council of Science and Technology of Mexico) and by the participating centers. Thuy Le reports grants from Gilead Sciences, outside the submitted work. B.J.M. reports grants from NIH/NHLBI, and from Bayer Pharmaceuticals, Inc., outside the submitted work. S.M. receives funding as the Innovative Medicines Canada Chair in Pandemic Preparedness. C.M. reports grants from the Health Research Council of New Zealand. M.J.M. reports having received the HCQ drug from the New York State government, during the conduct of the study; grants from Lilly, Pfizer, Sanofi, and personal fees from Meissa, outside the submitted work; in addition, M.J.M. has a patent anti-Zika monoclonal ab/ Emory Univ pending. A.N. is supported by a Health Research Board of Ireland Clinical Trial Network Award (HRB-CTH-2014-012). L.P. is an employee of Excelya and works as an external contractor for Sanofi. F.W.R. reports personal fees from Merck Research Labs, Novartis, Lilly, Sanofi, NovoNordisk, KLSMC, Tolerion, Rhythm, UCB, AstraZeneca, Janssen, Merck KGaA, Sarepta, Eidos, Amgen, Phathom, outside the submitted work; and having equity interest in GlaxoSmithkline, Athira Pharma, DataVant, Spencer Healthcare. S.R.W. reports receiving a grant from Sanofi during the conduct of the study and grants from NIH-NIAID outside the submitted work, and having conducted vaccine (HIV, Zika) clinical trials funded by Janssen. S.A.W. reports grants from National Health and Medical Research Council (Australia), grants from Minderoo Foundation, from Health Research Council (New Zealand), and from Medical Research Future Fund (Australia), during the conduct of the study. J.M.W. reports grants from ZonMw, Netherlands organisation for Health Research and Development, during the conduct of the study. F.G.Z. was part of the Coalition 1 trial partially supported by EMS Pharmaceuticals, has received previous grants from Bactiguard, Sweden, outside the submitted work, and support from Baxter LA for another clinical trial in critically ill patients. None of the other authors have any competing interests to declare.
Figures

References
-
- Berlin, D. A., Gulick, R. M. & Martinez, F. J. Severe Covid-19. N. Engl. J. Med.10.1056/NEJMcp2009575 (2020). - PubMed
-
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map. https://coronavirus.jhu.edu/map.html (2020).
-
- RECOVERY Collaborative Group et al. Dexamethasone in hospitalized Patients with Covid-19 - preliminary report. N. Engl. J. Med.10.1056/NEJMoa2021436 (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

