Optimising assessment of dark adaptation data using time to event analysis
- PMID: 33859209
- PMCID: PMC8050245
- DOI: 10.1038/s41598-021-86193-3
Optimising assessment of dark adaptation data using time to event analysis
Abstract
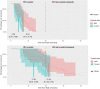
In age-related macular degeneration (AMD) research, dark adaptation has been found to be a promising functional measurement. In more severe cases of AMD, dark adaptation cannot always be recorded within a maximum allowed time for the test (~ 20-30 min). These data are recorded either as censored data-points (data capped at the maximum test time) or as an estimated recovery time based on the trend observed from the data recorded within the maximum recording time. Therefore, dark adaptation data can have unusual attributes that may not be handled by standard statistical techniques. Here we show time-to-event analysis is a more powerful method for analysis of rod-intercept time data in measuring dark adaptation. For example, at 80% power (at α = 0.05) sample sizes were estimated to be 20 and 61 with uncapped (uncensored) and capped (censored) data using a standard t-test; these values improved to 12 and 38 when using the proposed time-to-event analysis. Our method can accommodate both skewed data and censored data points and offers the advantage of significantly reducing sample sizes when planning studies where this functional test is an outcome measure. The latter is important because designing trials and studies more efficiently equates to newer treatments likely being examined more efficiently.
Conflict of interest statement
GM, DPC and AMB received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant 116076 (MACUSTAR). This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation program and European Federation of Pharmaceutical Industries and Associations (EFPIA). The communication reflects the authors’ view and that neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein. GM is a consultant for CenterVue and DPC is a consultant for CenterVue and Apellis, has received speaker’s fees from Santen, Allergan and Bayer, and has received compensation as a member of the scientific advisory board of Roche. These affiliations do not directly impact the work in this manuscript. Remaining author BEH declares that they have no conflict of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

