Transcriptional profiles in Strongyloides stercoralis males reveal deviations from the Caenorhabditis sex determination model
- PMID: 33859232
- PMCID: PMC8050236
- DOI: 10.1038/s41598-021-87478-3
Transcriptional profiles in Strongyloides stercoralis males reveal deviations from the Caenorhabditis sex determination model
Abstract
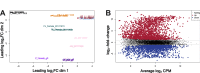
The human and canine parasitic nematode Strongyloides stercoralis utilizes an XX/XO sex determination system, with parasitic females reproducing by mitotic parthenogenesis and free-living males and females reproducing sexually. However, the genes controlling S. stercoralis sex determination and male development are unknown. We observed precocious development of rhabditiform males in permissive hosts treated with corticosteroids, suggesting that steroid hormones can regulate male development. To examine differences in transcript abundance between free-living adult males and other developmental stages, we utilized RNA-Seq. We found two clusters of S. stercoralis-specific genes encoding predicted transmembrane proteins that are only expressed in free-living males. We additionally identified homologs of several genes important for sex determination in Caenorhabditis species, including mab-3, tra-1, fem-2, and sex-1, which may have similar functions. However, we identified three paralogs of gld-1; Ss-qki-1 transcripts were highly abundant in adult males, while Ss-qki-2 and Ss-qki-3 transcripts were highly abundant in adult females. We also identified paralogs of pumilio domain-containing proteins with sex-specific transcripts. Intriguingly, her-1 appears to have been lost in several parasite lineages, and we were unable to identify homologs of tra-2 outside of Caenorhabditis species. Together, our data suggest that different mechanisms control male development in S. stercoralis and Caenorhabditis species.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

