Functional NMDA receptors are expressed by human pulmonary artery smooth muscle cells
- PMID: 33859248
- PMCID: PMC8050278
- DOI: 10.1038/s41598-021-87667-0
Functional NMDA receptors are expressed by human pulmonary artery smooth muscle cells
Abstract
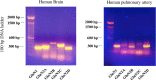
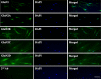
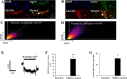
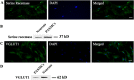
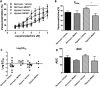
N-methyl-D-aspartate (NMDA) receptors are widely expressed in the central nervous system. However, their presence and function at extraneuronal sites is less well characterized. In the present study, we examined the expression of NMDA receptor subunit mRNA and protein in human pulmonary artery (HPA) by quantitative polymerase chain reaction (PCR), immunohistochemistry and immunoblotting. We demonstrate that both GluN1 and GluN2 subunit mRNAs are expressed in HPA. In addition, GluN1 and GluN2 (A-D) subunit proteins are expressed by human pulmonary artery smooth muscle cells (HPASMCs) in vitro and in vivo. These subunits localize on the surface of HPASMCs and form functional ion channels as evidenced by whole-cell patch-clamp electrophysiology and reduced phenylephrine-induced contractile responsiveness of human pulmonary artery by the NMDA receptor antagonist MK801 under hypoxic condition. HPASMCs also express high levels of serine racemase and vesicular glutamate transporter 1, suggesting a potential source of endogenous agonists for NMDA receptor activation. Our findings show HPASMCs express functional NMDA receptors in line with their effect on pulmonary vasoconstriction, and thereby suggest a novel therapeutic target for pharmacological modulations in settings associated with pulmonary vascular dysfunction.
Conflict of interest statement
The authors declare no competing interests.
Figures


![Figure 8[replace with the revised figure]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ebdf/8050278/18d9679a0903/41598_2021_87667_Fig8_HTML.gif)
References
-
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

