Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability
- PMID: 33859356
- PMCID: PMC8517038
- DOI: 10.1038/s41380-021-01093-2
Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability
Abstract
Ketamine, a racemic mixture of (S)-ketamine and (R)-ketamine enantiomers, has been used as an anesthetic, analgesic and more recently, as an antidepressant. However, ketamine has known abuse liability (the tendency of a drug to be used in non-medical situations due to its psychoactive effects), which raises concerns for its therapeutic use. (S)-ketamine was recently approved by the United States' FDA for treatment-resistant depression. Recent studies showed that (R)-ketamine has greater efficacy than (S)-ketamine in preclinical models of depression, but its clinical antidepressant efficacy has not been established. The behavioral effects of racemic ketamine have been studied extensively in preclinical models predictive of abuse liability in humans (self-administration and conditioned place preference [CPP]). In contrast, the behavioral effects of each enantiomer in these models are unknown. We show here that in the intravenous drug self-administration model, the gold standard procedure to assess potential abuse liability of drugs in humans, rats self-administered (S)-ketamine but not (R)-ketamine. Subanesthetic, antidepressant-like doses of (S)-ketamine, but not of (R)-ketamine, induced locomotor activity (in an opioid receptor-dependent manner), induced psychomotor sensitization, induced CPP in mice, and selectively increased metabolic activity and dopamine tone in medial prefrontal cortex (mPFC) of rats. Pharmacological screening across thousands of human proteins and at biological targets known to interact with ketamine yielded divergent binding and functional enantiomer profiles, including selective mu and kappa opioid receptor activation by (S)-ketamine in mPFC. Our results demonstrate divergence in the pharmacological, functional, and behavioral effects of ketamine enantiomers, and suggest that racemic ketamine's abuse liability in humans is primarily due to the pharmacological effects of its (S)-enantiomer.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Conflict of interest
All other authors declare no conflict of interest.
Figures


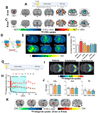
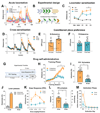
Comment in
-
Unraveling the opioid actions of S-ketamine and R-ketamine: comment on Bonaventura et al.Mol Psychiatry. 2021 Nov;26(11):6104-6106. doi: 10.1038/s41380-021-01167-1. Epub 2021 May 18. Mol Psychiatry. 2021. PMID: 34006965 No abstract available.
-
Comments to pharmacological and behavioral divergence of ketamine enantiomers by Jordi Bonaventura et al.Mol Psychiatry. 2022 Apr;27(4):1860-1862. doi: 10.1038/s41380-022-01447-4. Epub 2022 Feb 17. Mol Psychiatry. 2022. PMID: 35177823 Free PMC article. No abstract available.
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. , Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47, 351–354 (2000). - PubMed
-
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. , A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63, 856–864 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

