Microglia use TAM receptors to detect and engulf amyloid β plaques
- PMID: 33859405
- PMCID: PMC8102389
- DOI: 10.1038/s41590-021-00913-5
Microglia use TAM receptors to detect and engulf amyloid β plaques
Abstract
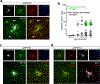
Two microglial TAM receptor tyrosine kinases, Axl and Mer, have been linked to Alzheimer's disease, but their roles in disease have not been tested experimentally. We find that in Alzheimer's disease and its mouse models, induced expression of Axl and Mer in amyloid plaque-associated microglia was coupled to induced plaque decoration by the TAM ligand Gas6 and its co-ligand phosphatidylserine. In the APP/PS1 mouse model of Alzheimer's disease, genetic ablation of Axl and Mer resulted in microglia that were unable to normally detect, respond to, organize or phagocytose amyloid-β plaques. These major deficits notwithstanding, TAM-deficient APP/PS1 mice developed fewer dense-core plaques than APP/PS1 mice with normal microglia. Our findings reveal that the TAM system is an essential mediator of microglial recognition and engulfment of amyloid plaques and that TAM-driven microglial phagocytosis does not inhibit, but rather promotes, dense-core plaque development.
Conflict of interest statement
Figures



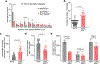



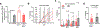
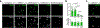

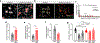

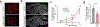
Comment in
-
TAM-ping down amyloid in Alzheimer's disease.Nat Immunol. 2021 May;22(5):543-544. doi: 10.1038/s41590-021-00918-0. Nat Immunol. 2021. PMID: 33859407 Free PMC article.
-
Dense-core plaques could be beneficial in AD.Nat Rev Neurol. 2021 Jun;17(6):328. doi: 10.1038/s41582-021-00513-9. Nat Rev Neurol. 2021. PMID: 33986528 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

