Immunoassay-Amplified Responses Using a Functionalized MoS2-Based SPR Biosensor to Detect PAPP-A2 in Maternal Serum Samples to Screen for Fetal Down's Syndrome
- PMID: 33859474
- PMCID: PMC8043798
- DOI: 10.2147/IJN.S296406
Immunoassay-Amplified Responses Using a Functionalized MoS2-Based SPR Biosensor to Detect PAPP-A2 in Maternal Serum Samples to Screen for Fetal Down's Syndrome
Abstract
Background: Due to educational, social and economic reasons, more and more women are delaying childbirth. However, advanced maternal age is associated with several adverse pregnancy outcomes, and in particular a high risk of Down's syndrome (DS). Hence, it is increasingly important to be able to detect fetal Down's syndrome (FDS).
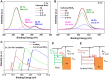
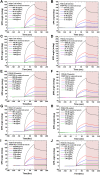
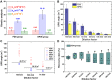
Methods: We developed an effective, highly sensitive, surface plasmon resonance (SPR) biosensor with biochemically amplified responses using carboxyl-molybdenum disulfide (MoS2) film. The use of carboxylic acid as a surface modifier of MoS2 promoted dispersion and formed specific three-dimensional coordination sites. The carboxylic acid immobilized unmodified antibodies in a way that enhanced the bioaffinity of MoS2 and preserved biorecognition properties of the SPR sensor surface. Complete antigen pregnancy-associated plasma protein-A2 (PAPP-A2) conjugated with the carboxyl-MoS2-modified gold chip to amplify the signal and improve detection sensitivity. This heterostructure interface had a high work function, and thus improved the efficiency of the electric field energy of the surface plasmon. These results provide evidence that the interface electric field improved performance of the SPR biosensor.
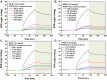
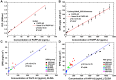
Results: The carboxyl-MoS2-based SPR biosensor was used successfully to evaluate PAPP-A2 level for fetal Down's syndrome screening in maternal serum samples. The detection limit was 0.05 pg/mL, and the linear working range was 0.1 to 1100 pg/mL. The women with an SPR angle >46.57 m° were more closely associated with fetal Down's syndrome. Once optimized for serum Down's syndrome screening, an average recovery of 95.2% and relative standard deviation of 8.5% were obtained. Our findings suggest that carboxyl-MoS2-based SPR technology may have advantages over conventional ELISA in certain situations.
Conclusion: Carboxyl-MoS2-based SPR biosensors can be used as a new diagnostic technology to respond to the increasing need for fetal Down's syndrome screening in maternal serum samples. Our results demonstrated that the carboxyl-MoS2-based SPR biosensor was capable of determining PAPP-A2 levels with acceptable accuracy and recovery. We hope that this technology will be investigated in diverse clinical trials and in real case applications for screening and early diagnosis in the future.
Keywords: DS; Down’s syndrome; FDS; PAPP-A2; SPR; carboxyl-MoS2; carboxyl-functionalized molybdenum disulfide; fetal Down’s syndrome; pregnancy-associated plasma protein A2; surface plasmon resonance.
© 2021 Chiu et al.
Conflict of interest statement
Prof. Dr. Nan-Fu Chiu reports a patent US10634613B2 issued, a patent US10815259B2. The authors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

