Clinical Outcome and Cost-Effectiveness Analysis of CSII Versus MDI in Children and Adolescent With Type 1 Diabetes Mellitus in a Public Health Care System of China
- PMID: 33859614
- PMCID: PMC8043415
- DOI: 10.3389/fendo.2021.604028
Clinical Outcome and Cost-Effectiveness Analysis of CSII Versus MDI in Children and Adolescent With Type 1 Diabetes Mellitus in a Public Health Care System of China
Abstract
Objectives: To evaluate the clinical and economic consequences of continuous subcutaneous insulin infusion (CSII) vs. multiple daily injections (MDI) in children and adolescents with type 1 diabetes mellitus (T1DM) from a public health care system in developed areas of developing country, considering changes in glycemic Control, daily insulin requirements, lipid profile, body mass index (BMI), frequency of severe hypoglycemia and Diabetic Ketoacidosis (DKA) and diabetic complications.
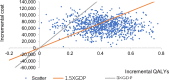
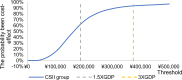
Methods: This was a retrospective cohort study of children and adolescents with T1DM. Data were collected at baseline and the end of every year including glycated hemoglobin (HbA1c), insulin dose, lipid profile, blood pressure, and adverse events (severe hypoglycemia and DKA). The Cost-effectiveness analysis was performed using the IQVIA CORE Diabetes Model (CDM) to simulate diabetes progression by utilizing the clinical data obtained from the two groups. The main outcome measures were Life Expectancy, Quality adjusted life years (QALYs), Total Costs and Incremental Costs and Effectiveness Ratio (ICER) of CSII compared with MDI in Chinese pediatric patients with T1DM in Qingdao City (60 years).
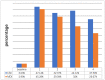
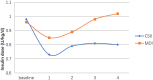
Results: Mean HbA1c values and daily insulin doses were significantly lower in those receiving CSII therapy throughout follow-up. Mean direct lifetime costs were ¥ 67,137 higher with CSII treatment than with MDI for pediatric patients. Treatment with CSII was associated with an improvement in life expectancy of 0.41 years for pediatric patients compared with MDI based on CORE diabetes model simulation. The corresponding gains in QALYs were 0.42. These data produced corresponding ICER is ¥ 161,815 per QALY for pediatric T1DM patients in Qingdao. Sensitivity analyses suggested that our base-case assumptions were mostly robust.
Conclusions: CSII is associated with improved long-term clinical outcomes compared with MDI. Based on this model analysis, CSII appears to be more cost-effective for the Qingdao TIDM pediatric population and health care system.
Keywords: continuous subcutaneous insulin infusion; cost-effectiveness analysis; glycated hemoglobin; incremental costs and effectiveness ratio; multiple daily injections; quality adjusted life years; type 1 diabetes mellitus.
Copyright © 2021 Hu, Yang, Chen, Leng, Li, Qiao, Lv and Li.
Conflict of interest statement
Author WL was employed by Medtronic (Shanghai) Management Co, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia.Pharmacoeconomics. 2007;25(10):881-97. doi: 10.2165/00019053-200725100-00006. Pharmacoeconomics. 2007. PMID: 17887808
-
Continuous subcutaneous insulin infusion versus multiple daily injections in children and young people at diagnosis of type 1 diabetes: the SCIPI RCT.Health Technol Assess. 2018 Aug;22(42):1-112. doi: 10.3310/hta22420. Health Technol Assess. 2018. PMID: 30109847 Free PMC article. Clinical Trial.
-
Health economic comparison between continuous subcutaneous insulin infusion and multiple daily injections of insulin for the treatment of adult type 1 diabetes in Canada.Clin Ther. 2009 Mar;31(3):657-67. doi: 10.1016/j.clinthera.2009.03.013. Clin Ther. 2009. PMID: 19393856
-
Cost-effectiveness of continuous subcutaneous insulin infusion versus multiple daily injections of insulin in Type 1 diabetes: a systematic review.Diabet Med. 2015 Nov;32(11):1415-24. doi: 10.1111/dme.12792. Epub 2015 May 28. Diabet Med. 2015. PMID: 25962621
-
Cost-effectiveness of continuous subcutaneous insulin infusion (CSII) in children: illusion or delusion?Pediatr Diabetes. 2006 Aug;7 Suppl 4:39-44. doi: 10.1111/j.1399-543X.2006.00168.x. Pediatr Diabetes. 2006. PMID: 16774617 Review.
Cited by
-
MDI versus CSII in Chinese adults with type 1 diabetes in a real-world situation: based on propensity score matching method.Health Qual Life Outcomes. 2024 Jun 13;22(1):47. doi: 10.1186/s12955-024-02263-w. Health Qual Life Outcomes. 2024. PMID: 38872219 Free PMC article.
-
The impact of sodium glucose co-transporter 2 inhibitors and glucagon-like peptide 1 receptor agonists on insulin utilisation and costs in Australia: a national retrospective observational cross-sectional study.Lancet Reg Health West Pac. 2024 Sep 24;52:101207. doi: 10.1016/j.lanwpc.2024.101207. eCollection 2024 Nov. Lancet Reg Health West Pac. 2024. PMID: 39381086 Free PMC article.
-
Comparing the effectiveness of continuous subcutaneous insulin infusion with multiple daily insulin injection for patients with type 1 diabetes mellitus evaluated by retrospective continuous glucose monitoring: A real-world data analysis.Front Public Health. 2022 Aug 25;10:990281. doi: 10.3389/fpubh.2022.990281. eCollection 2022. Front Public Health. 2022. PMID: 36091534 Free PMC article.
-
Effect of the Chinese New Year Public Holiday on the Glycemic Control of T1DM With Intensive Insulin Therapy.Front Endocrinol (Lausanne). 2022 Jun 28;13:915482. doi: 10.3389/fendo.2022.915482. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837316 Free PMC article.
-
Factors influencing insulin requirements in using continuous subcutaneous insulin infusion or multiple daily injections in type 2 diabetes.World J Diabetes. 2025 Jul 15;16(7):106470. doi: 10.4239/wjd.v16.i7.106470. World J Diabetes. 2025. PMID: 40697604 Free PMC article. Clinical Trial.
References
-
- Subspecialty Group of Endocrinologic. Hereditary and Metabolic Diseases, the Society of Pediatrics. Chinese Medical Association, Editorial Board. Chinese Journal of Pediatrics . Expert Consensus on the Standardized Diagnosis and Management of Type 1 Diabetes Mellitus in Chinese Children (2020). Zhonghua Er Ke Za Zhi (2020) 58:447–54. 10.3760/cma.j.cn112140-20200221-00124 - DOI - PubMed
-
- Tauschmann M, Hermann JM, Freiberg C, Papsch M, Thon A, Heidtmann B, et al. . Reduction in Diabetic Ketoacidosis and Severe Hypoglycemia in Pediatric Type 1 Diabetes During the First Year of Continuous Glucose Monitoring: A Multicenter Analysis of 3,553 Subjects From the DPV Registry. Diabetes Care (2020) 3:e40–2. 10.2337/dc19-1358 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

