Activity and Safety of Tegafur, Gimeracil, and Oteracil Potassium for Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis
- PMID: 33859690
- PMCID: PMC8009729
- DOI: 10.1155/2021/6690275
Activity and Safety of Tegafur, Gimeracil, and Oteracil Potassium for Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis
Abstract
In clinical practice, tegafur, gimeracil, and oteracil potassium (S-1) therapy is commonly administered to treat nasopharyngeal carcinoma (NPC). However, its efficacy and safety remain controversial in both randomized controlled trials (RCTs) and non-RCTs. We aimed to evaluate the efficacy and safety of S-1 treatment for NPC. We searched PubMed, Ovid, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, and VIP databases for RCTs of chemotherapy with or without S-1 for NPC, from 2001 to 2020. A meta-analysis was performed using RevMan5.3 and Stata15. Randomized controlled trials published in journals were included irrespective of blinding and language used. Patients were diagnosed with NPC through a clinicopathological examination; patients of all cancer stages and ages were included. Overall, 25 trials and 1858 patients were included. There were significant differences in the complete remission (OR = 2.42, 95% CI (1.88-3.10), P < 0.05) and overall response rate (OR = 2.68, 95% CI (2.08-3.45), P < 0.05) between the S-1 and non-S-1 groups. However, there was no significant difference in partial remission (OR = 1.10, 95% CI (0.87-1.39), P=0.42) and seven adverse reactions (leukopenia, thrombocytopenia, nausea and vomiting, diarrhea, dermatitis, oral mucositis, and anemia) between the S-1 and non-S-1 groups. Additionally, statistical analyses with six subgroups were performed. S-1 was found to be a satisfactory chemotherapeutic agent combined with radiotherapy, intravenous chemotherapy, or chemoradiotherapy for NPC. As an oral medicine, the adverse reactions of S-1, especially gastrointestinal reactions, can be tolerated by patients, thereby optimizing their quality of life. S-1 may be a better choice for the treatment of NPC. This trial is registered with CRD42019122041.
Copyright © 2021 Ximing Zhang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

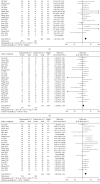

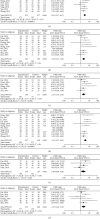

Similar articles
-
Effects of S-1 Combined with Radiotherapy in the Treatment of Nasopharyngeal Cancer: A Meta-analysis Based on Randomized Controlled Trials.Open Med (Wars). 2017 May 11;12:107-114. doi: 10.1515/med-2017-0017. eCollection 2017. Open Med (Wars). 2017. PMID: 28730169 Free PMC article.
-
Efficacy and safety of S-1 (tegafur, gimeracil, and oteracil potassium) concurrent with 3-dimensional conformal radiotherapy for newly diagnosed squamous cell carcinoma of the lung in elderly patients.Cancer Radiother. 2016 May;20(3):181-6. doi: 10.1016/j.canrad.2015.12.004. Epub 2016 Apr 7. Cancer Radiother. 2016. PMID: 27068497 Clinical Trial.
-
The Clinical Evaluation of Tegafur Gimeracil Oteracil Combined with THP and DDP for Second-Line Treatment of Advanced Cardiac Carcinoma.Cell Biochem Biophys. 2015 Jul;72(3):695-9. doi: 10.1007/s12013-015-0520-0. Cell Biochem Biophys. 2015. PMID: 25618173 Clinical Trial.
-
Kangai Injection Combined with Platinum-based Chemotherapy for the Treatment of Stage III/IV Non-Small Cell Lung Cancer: A Meta-analysis and Systematic Review of 35 Randomized Controlled Trials.J Cancer. 2019 Aug 28;10(21):5283-5298. doi: 10.7150/jca.31928. eCollection 2019. J Cancer. 2019. PMID: 31602279 Free PMC article. Review.
-
Efficacy of traditional Chinese Medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): a meta-analysis of randomized clinical trials.Support Care Cancer. 2020 Aug;28(8):3571-3579. doi: 10.1007/s00520-020-05433-w. Epub 2020 Apr 7. Support Care Cancer. 2020. PMID: 32266566
Cited by
-
Platinum Plus Tegafur-Uracil versus Platinum Alone during Concurrent Chemoradiotherapy in Patients with Nonmetastatic Nasopharyngeal Carcinoma: A Propensity-Score-Matching Analysis.Cancers (Basel). 2022 Sep 17;14(18):4511. doi: 10.3390/cancers14184511. Cancers (Basel). 2022. PMID: 36139674 Free PMC article.
-
Is Immune Therapy Plus Chemotherapy More Effective Than Immune Therapy Alone for Unresectable Recurrent Nasopharyngeal Carcinoma?Front Immunol. 2021 Oct 29;12:762663. doi: 10.3389/fimmu.2021.762663. eCollection 2021. Front Immunol. 2021. PMID: 34777379 Free PMC article. Clinical Trial.
-
Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds-Part 1: Aromatic Frameworks.Molecules. 2025 Aug 4;30(15):3264. doi: 10.3390/molecules30153264. Molecules. 2025. PMID: 40807439 Free PMC article. Review.
-
The use of adjuvant chemotherapy combined with concurrent chemoradiotherapy enhances survival rates in cases of locally advanced nasopharyngeal carcinoma.Am J Cancer Res. 2024 Jun 15;14(6):3142-3152. doi: 10.62347/WMLA4979. eCollection 2024. Am J Cancer Res. 2024. PMID: 39005679 Free PMC article.
References
-
- Han Y. Q., He Q., Liu F., et al. A study of TPS1 and TPF neoadjuvant chemotherapy on curative effect for treatment of patients with locally advanced nasopharyngeal carcinoma. Anti-Tumor Pharmacy. 2017;7:425–430.
-
- Zhang M.-X., Li J., Shen G.-P., et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. European Journal of Cancer. 2015;51(17):2587–2595. doi: 10.1016/j.ejca.2015.08.006. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

