Crataegus pinnatifida Bunge Inhibits RANKL-Induced Osteoclast Differentiation in RAW 264.7 Cells and Prevents Bone Loss in an Ovariectomized Rat Model
- PMID: 33859705
- PMCID: PMC8024084
- DOI: 10.1155/2021/5521562
Crataegus pinnatifida Bunge Inhibits RANKL-Induced Osteoclast Differentiation in RAW 264.7 Cells and Prevents Bone Loss in an Ovariectomized Rat Model
Abstract
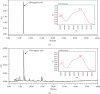
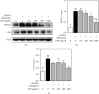
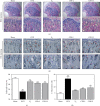
Osteoporosis is characterized by a decrease in bone microarchitecture with an increased risk of fracture. Long-term use of primary treatments, such as bisphosphonates and selective estrogen receptor modulators, results in various side effects. Therefore, it is necessary to develop alternative therapeutics derived from natural products. Crataegus pinnatifida Bunge (CPB) is a dried fruit used to treat diet-induced indigestion, loss of appetite, and diarrhea. However, research into the effects of CPB on osteoclast differentiation and osteoporosis is still limited. In vitro experiments were conducted to examine the effects of CPB on RANKL-induced osteoclast differentiation in RAW 264.7 cells. Moreover, we investigated the effects of CPB on bone loss in the femoral head in an ovariectomized rat model using microcomputed tomography. In vitro, tartrate-resistant acid phosphatase (TRAP) staining results showed the number of TRAP-positive cells, and TRAP activity significantly decreased following CPB treatment. CPB also significantly decreased pit formation. Furthermore, CPB inhibited osteoclast differentiation by suppressing NFATc1, and c-Fos expression. Moreover, CPB treatment inhibited osteoclast-related genes, such as Nfatc1, Ca2, Acp5, mmp9, CtsK, Oscar, and Atp6v0d2. In vivo, bone mineral density and structure model index were improved by administration of CPB. In conclusion, CPB prevented osteoclast differentiation in vitro and prevented bone loss in vivo. Therefore, CPB could be a potential alternative medicine for bone diseases, such as osteoporosis.
Copyright © 2021 Minsun Kim et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Chaenomelis fructus inhibits osteoclast differentiation by suppressing NFATc1 expression and prevents ovariectomy-induced osteoporosis.BMC Complement Med Ther. 2020 Feb 5;20(1):35. doi: 10.1186/s12906-020-2841-9. BMC Complement Med Ther. 2020. PMID: 32024503 Free PMC article.
-
Longan fruit increase bone mineral density in zebrafish and ovariectomized rat by suppressing RANKL-induced osteoclast differentiation.Phytomedicine. 2019 Jun;59:152910. doi: 10.1016/j.phymed.2019.152910. Epub 2019 Apr 2. Phytomedicine. 2019. PMID: 30978650
-
Berberine Suppresses RANKL-Induced Osteoclast Differentiation by Inhibiting c-Fos and NFATc1 Expression.Am J Chin Med. 2019;47(2):439-455. doi: 10.1142/S0192415X19500228. Epub 2019 Mar 4. Am J Chin Med. 2019. PMID: 30827151
-
Cyperus Rotundus L. extract suppresses RANKL-induced osteoclastogenesis through NFATc1/c-fos downregulation and prevent bone loss in OVX-induced osteoporosis rat.J Ethnopharmacol. 2017 Jun 9;205:186-194. doi: 10.1016/j.jep.2017.03.017. Epub 2017 Mar 15. J Ethnopharmacol. 2017. PMID: 28315458
-
Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+ -calcineurin-NFATc1 pathway.J Cell Physiol. 2019 May;234(5):6831-6841. doi: 10.1002/jcp.27432. Epub 2018 Nov 1. J Cell Physiol. 2019. PMID: 30387123
Cited by
-
Solanum nigrum Line inhibits osteoclast differentiation and suppresses bone mineral density reduction in the ovariectomy‑induced osteoporosis model.Mol Med Rep. 2021 Aug;24(2):607. doi: 10.3892/mmr.2021.12246. Epub 2021 Jun 29. Mol Med Rep. 2021. PMID: 34184079 Free PMC article.
-
Effects of Melandrium firmum Rohrbach on RANKL‑induced osteoclast differentiation and OVX rats.Mol Med Rep. 2021 Aug;24(2):610. doi: 10.3892/mmr.2021.12248. Epub 2021 Jun 29. Mol Med Rep. 2021. PMID: 34184080 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

