Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma
- PMID: 33859758
- PMCID: PMC8039945
- DOI: 10.7150/thno.54822
Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma
Abstract
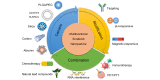
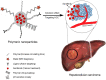
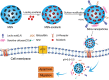
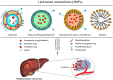
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and one of the leading causes of cancer-related death worldwide. Advanced HCC displays strong resistance to chemotherapy, and traditional chemotherapy drugs do not achieve satisfactory therapeutic efficacy. Sorafenib is an oral kinase inhibitor that inhibits tumor cell proliferation and angiogenesis and induces cancer cell apoptosis. It also improves the survival rates of patients with advanced liver cancer. However, due to its poor solubility, fast metabolism, and low bioavailability, clinical applications of sorafenib have been substantially restricted. In recent years, various studies have been conducted on the use of nanoparticles to improve drug targeting and therapeutic efficacy in HCC. Moreover, nanoparticles have been extensively explored to improve the therapeutic efficacy of sorafenib, and a variety of nanoparticles, such as polymer, lipid, silica, and metal nanoparticles, have been developed for treating liver cancer. All these new technologies have improved the targeted treatment of HCC by sorafenib and promoted nanomedicines as treatments for HCC. This review provides an overview of hot topics in tumor nanoscience and the latest status of treatments for HCC. It further introduces the current research status of nanoparticle drug delivery systems for treatment of HCC with sorafenib.
Keywords: Hepatocellular carcinoma; Nanomaterials; Nanomedicine.; Nanoparticles; Sorafenib.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Villanueva A. Hepatocellular Carcinoma. The New England journal of medicine. 2019;380:1450–62. - PubMed
-
- Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48:7–17. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

