Increase in Blood-Brain Barrier (BBB) Permeability Is Regulated by MMP3 via the ERK Signaling Pathway
- PMID: 33859779
- PMCID: PMC8026308
- DOI: 10.1155/2021/6655122
Increase in Blood-Brain Barrier (BBB) Permeability Is Regulated by MMP3 via the ERK Signaling Pathway
Abstract
Background: The blood-brain barrier (BBB) regulates the exchange of molecules between the brain and peripheral blood and is composed primarily of microvascular endothelial cells (BMVECs), which form the lining of cerebral blood vessels and are linked via tight junctions (TJs). The BBB is regulated by components of the extracellular matrix (ECM), and matrix metalloproteinase 3 (MMP3) remodels the ECM's basal lamina, which forms part of the BBB. Oxidative stress is implicated in activation of MMPs and impaired BBB. Thus, we investigated whether MMP3 modulates BBB permeability.
Methods: Experiments included in vivo assessments of isoflurane anesthesia and dye extravasation from brain in wild-type (WT) and MMP3-deficient (MMP3-KO) mice, as well as in vitro assessments of the integrity of monolayers of WT and MMP3-KO BMVECs and the expression of junction proteins.
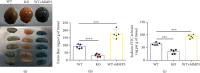
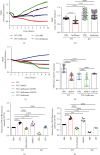
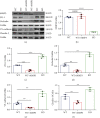
Results: Compared to WT mice, measurements of isoflurane usage and anesthesia induction time were higher in MMP3-KO mice and lower in WT that had been treated with MMP3 (WT+MMP3), while anesthesia emergence times were shorter in MMP3-KO mice and longer in WT+MMP3 mice than in WT. Extravasation of systemically administered dyes was also lower in MMP3-KO mouse brains and higher in WT+MMP3 mouse brains, than in the brains of WT mice. The results from both TEER and Transwell assays indicated that MMP3 deficiency (or inhibition) increased, while MMP3 upregulation reduced barrier integrity in either BMVEC or the coculture. MMP3 deficiency also increased the abundance of TJs and VE-cadherin proteins in BMVECs, and the protein abundance declined when MMP3 activity was upregulated in BMVECs, but not when the cells were treated with an inhibitor of extracellular signal related-kinase (ERK).
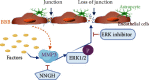
Conclusion: MMP3 increases BBB permeability following the administration of isoflurane by upregulating the ERK signaling pathway, which subsequently reduces TJ and VE-cadherin proteins in BMVECs.
Copyright © 2021 Qin Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures




References
-
- Chen J. T., Lin Y. L., Chen T. L., Tai Y. T., Chen C. Y., Chen R. M. Ketamine alleviates bradykinin-induced disruption of the mouse cerebrovascular endothelial cell-constructed tight junction barrier via a calcium-mediated redistribution of occludin polymerization. Toxicology. 2016;368-369:142–151. doi: 10.1016/j.tox.2016.09.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

