Discovery of a Highly Selective and Potent TRPC3 Inhibitor with High Metabolic Stability and Low Toxicity
- PMID: 33859797
- PMCID: PMC8040052
- DOI: 10.1021/acsmedchemlett.0c00571
Discovery of a Highly Selective and Potent TRPC3 Inhibitor with High Metabolic Stability and Low Toxicity
Abstract
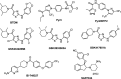
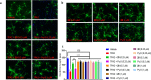
The overactivation of transient receptor potential canonical 3 (TRPC3) is associated with neurodegenerative diseases and hypertension. Pyrazole 3 (Pyr3) is reported as the most selective TRPC3 inhibitor, but it has two inherent structural limitations: (1) the labile ester moiety leads to its rapid hydrolysis to the inactive Pyr8 in vivo, and (2) the alkylating trichloroacrylic amide moiety is known to be toxic. To circumvent these limitations, we designed a series of conformationally restricted Pyr3 analogues and reported that compound 20 maintains high potency and selectivity for human TRPC3 over its closely related TRP channels. It has significantly improved metabolic stability compared with Pyr3 and has a good safety profile. Preliminary evaluation of 20 demonstrated its ability to rescue Aβ-induced neuron damage with similar potency to that of Pyr3 in vitro. Collectively, these results suggest that 20 represents a promising scaffold to potentially ameliorate the symptoms associated with TRPC3-mediated neurological and cardiovascular disorders.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): W.L., Z.W., and F.L. are listed as inventors for a filed provisional patent application covering these compounds.
Figures
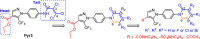






References
-
- Mori Y.; Wakamori M.; Miyakawa T.; Hermosura M.; Hara Y.; Nishida M.; Hirose K.; Mizushima A.; Kurosaki M.; Mori E.; Gotoh K.; Okada T.; Fleig A.; Penner R.; Iino M.; Kurosaki T. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J. Exp. Med. 2002, 195, 673–681. 10.1084/jem.20011758. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

