Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group
- PMID: 33859802
- PMCID: PMC8040035
- DOI: 10.1021/acsmedchemlett.1c00024
Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group
Abstract
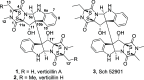
The verticillins, a class of epipolythiodioxopiperazine alkaloids (ETPs) first described 50 years ago with the discovery of verticillin A (1), have gained attention due to their potent activity against cancer cells, noted both in vitro and in vivo. In this study, the complex scaffold afforded through optimized fermentation was used as a feedstock for semisynthetic efforts designed to explore the reactivity of the C11 and C11' hydroxy substituents. Functionality introduced at these positions would be expected to impact not only the potency but also the pharmacokinetic properties of the resulting compound. With this in mind, verticillin H (2) was used as a starting material to generate nine semisynthetic analogues (4-12) containing a variety of ester, carbonate, carbamate, and sulfonate moieties. Likewise, verticillin A succinate (13) was synthesized from 1 to demonstrate the successful application of this strategy to other ETPs. The synthesized compounds and their corresponding starting materials (i.e., 1 and 2) were screened for activity against a panel of melanoma, breast, and ovarian cancer cell lines: MDA-MB-435, MDA-MB-231, and OVCAR3. All analogues retained IC50 values in the nanomolar range, comparable to, and in some cases more potent than, the parent compounds.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Nicholas Oberlies is a member of the Scientific Advisory Board of Mycosynthetix, Inc.
Figures
References
-
- American Cancer Society . Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, 2019.
-
- Cragg G. M.; Newman D. J.. Natural Products as Sources of Anticancer Agents: Current Approaches and Perspectives. In Natural Products as Source of Molecules with Therapeutic Potential; Springer: 2018; pp 309–331.
-
- Kinghorn A. D.; De Blanco E. J. C.; Lucas D. M.; Rakotondraibe H. L.; Orjala J.; Soejarto D. D.; Oberlies N. H.; Pearce C. J.; Wani M. C.; Stockwell B. R.; Burdette J. E.; Swanson S. M.; Fuchs J. R.; Phelps M. A.; Xu L.; Zhang X.; Shen Y. Y. Discovery of anticancer agents of diverse natural origin. Anticancer Res. 2016, 36, 5623–5638. 10.21873/anticanres.11146. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

