Discovery of Diaminopyrimidine Carboxamide HPK1 Inhibitors as Preclinical Immunotherapy Tool Compounds
- PMID: 33859804
- PMCID: PMC8040257
- DOI: 10.1021/acsmedchemlett.1c00096
Discovery of Diaminopyrimidine Carboxamide HPK1 Inhibitors as Preclinical Immunotherapy Tool Compounds
Abstract
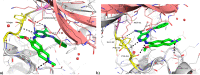
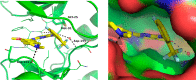
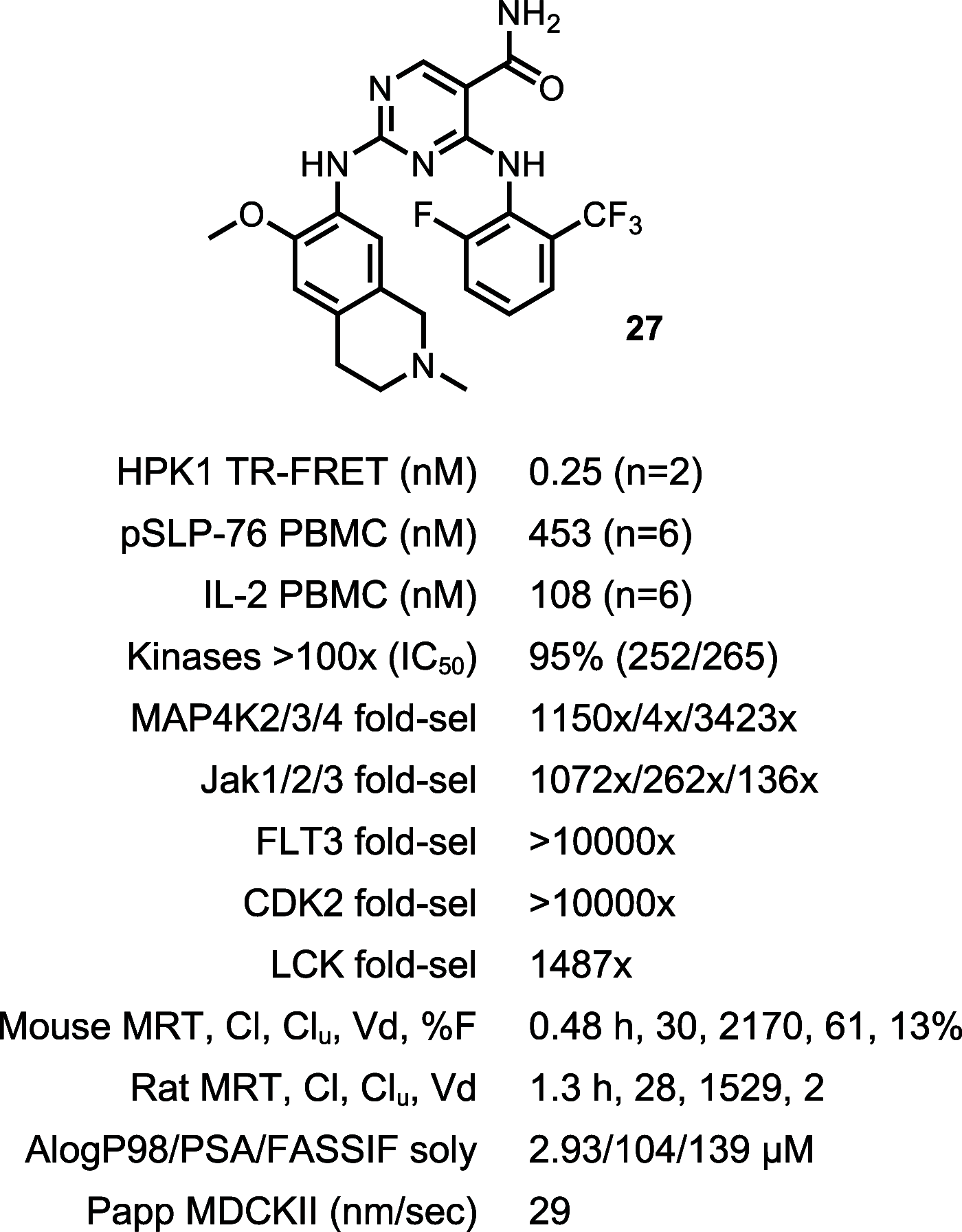
Hematopoietic progenitor kinase 1 (HPK1), a serine/threonine kinase, is a negative immune regulator of T cell receptor (TCR) and B cell signaling that is primarily expressed in hematopoietic cells. Accordingly, it has been reported that HPK1 loss-of-function in HPK1 kinase-dead syngeneic mouse models shows enhanced T cell signaling and cytokine production as well as tumor growth inhibition in vivo, supporting its value as an immunotherapeutic target. Herein, we present the structurally enabled discovery of novel, potent, and selective diaminopyrimidine carboxamide HPK1 inhibitors. The key discovery of a carboxamide moiety was essential for enhanced enzyme inhibitory potency and kinome selectivity as well as sustained elevation of cellular IL-2 production across a titration range in human peripheral blood mononuclear cells. The elucidation of structure-activity relationships using various pendant amino ring systems allowed for the identification of several small molecule type-I inhibitors with promising in vitro profiles.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare competing financial interests as Merck & Co., Inc., Kenilworth, NJ, USA stakeholders, stockholders and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA employees.
Figures

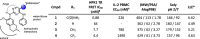


References
-
- Larkin J.; Chiarion-Sileni V.; Gonzalez R.; Grob J. J.; Cowey C. L.; Lao C. D.; Schadendorf D.; Dummer R.; Smylie M.; Rutkowski P. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. 10.1056/NEJMoa1504030. - DOI - PMC - PubMed
- McDermott D. F.; Drake C. G.; Sznol M.; Choueiri T. K.; Powderly J. D.; Smith D. C.; Brahmer J. R.; Carvajal R. D.; Hammers H. J.; Puzanov I. Survival, Durable Response, and Long-Term Safety in Patients with Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J. Clin. Oncol. 2015, 33, 2013.10.1200/JCO.2014.58.1041. - DOI - PMC - PubMed
- Ribas A.; Puzanov I.; Dummer R.; Schadendorf D.; Hamid O.; Robert C.; Hodi F. S.; Schachter J.; Pavlick A. C.; Lewis K. D. Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2015, 16, 908–918. 10.1016/S1470-2045(15)00083-2. - DOI - PMC - PubMed
- Topalian S. L.; Hodi F. S.; Brahmer J. R.; Gettinger S. N.; Smith D. C.; McDermott D. F.; Powderly J. D.; Sosman J. A.; Atkins M. B.; Leming P. D. Five-Year Survival and Correlates among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. 10.1001/jamaoncol.2019.2187. - DOI - PMC - PubMed
-
- Hodi F. S.; O’Day S. J.; McDermott D. F.; Weber R. W.; Sosman J. A.; Haanen J. B.; Gonzalez R.; Robert C.; Schadendorf D.; Hassel J. C. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
- Schadendorf D.; Hodi F. S.; Robert C.; Weber J. S.; Margolin K.; Hamid O.; Patt D.; Chen T.-T.; Berman D. M.; Wolchok J. D. Pooled Analysis of Long-Term Survival Data from Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889.10.1200/JCO.2014.56.2736. - DOI - PMC - PubMed
- Maio M.; Grob J.-J.; Aamdal S.; Bondarenko I.; Robert C.; Thomas L.; Garbe C.; Chiarion-Sileni V.; Testori A.; Chen T.-T. Five-Year Survival Rates for Treatment-Naive Patients with Advanced Melanoma Who Received Ipilimumab plus Dacarbazine in a Phase III Trial. J. Clin. Oncol. 2015, 33, 1191.10.1200/JCO.2014.56.6018. - DOI - PMC - PubMed
-
- Hashimoto M.; Kamphorst A. O.; Im S.j.; Kissick H. T.; Pillai R. N.; Ramalingam S. S.; Araki K.; Ahmed R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 2018, 69, 301–318. 10.1146/annurev-med-012017-043208. - DOI - PubMed
- Barber D. L.; Wherry E. J.; Masopust D.; Zhu B.; Allison J. P.; Sharpe A. H.; Freeman G. J.; Ahmed R. Restoring Function in Exhausted CD8 T Cells during Chronic Viral Infection. Nature 2006, 439, 682–687. 10.1038/nature04444. - DOI - PubMed
- Sharpe A. H. Introduction to Checkpoint Inhibitors and Cancer Immunotherapy. Immunol. Rev. 2017, 276, 5.10.1111/imr.12531. - DOI - PMC - PubMed
-
- Topalian S. L.; Drake C. G.; Pardoll D. M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. 10.1016/j.ccell.2015.03.001. - DOI - PMC - PubMed
- Mellman I.; Coukos G.; Dranoff G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. 10.1038/nature10673. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

