Tumor Lysis Syndrome: Introduction of a Cutaneous Variant and a New Classification System
- PMID: 33859885
- PMCID: PMC8038896
- DOI: 10.7759/cureus.13816
Tumor Lysis Syndrome: Introduction of a Cutaneous Variant and a New Classification System
Abstract
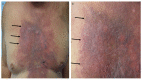
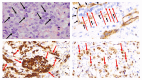
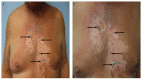
Tumor lysis syndrome, an oncological emergency, is characterized by laboratory parameters such as hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia, as well as renal injury with an elevated creatinine. Tumor lysis syndrome is seen in patients with aggressive malignancies and high tumor burden. More frequently, it occurs in individuals with hematologic malignancies such as high-grade lymphomas (such as Burkitt lymphoma) and leukemia (such as acute lymphocytic leukemia). It also, albeit less commonly, can be seen in patients with widespread solid tumors that are rapidly proliferating and are markedly sensitivity to antineoplastic therapy. Tumor lysis syndrome is usually preceded by cancer-directed therapy; however, the syndrome can present spontaneously prior to the individual receiving malignancy-directed treatment. We reported a man with metastatic salivary duct carcinoma who had cutaneous metastases that presented as carcinoma hemorrhagiectoides. Microscopic examination demonstrated that the metastatic tumor cells had infiltrated and replaced the entire dermis. After the patient received his first dose of antineoplastic therapy, he had an excellent response and the cutaneous metastases developed into ulcers; we hypothesize that most of the dermis, which had been replaced by tumor cells, disappeared as a result of the therapeutic response, and the overlying epidermis became necrotic and shed, leaving an ulcer. His dramatic response to treatment prompted us to propose a new classification of tumor lysis syndrome, which should include the systemic form of the condition as well as the new variant: cutaneous tumor lysis syndrome. We anticipate that, with improvement in targeted therapies, there may be an increase in therapy-associated cutaneous tumor lysis syndrome.
Keywords: cancer; carcinoma; cutaneous; hematologic; lysis; malignancy; metastatic; solid; syndrome; tumor.
Copyright © 2021, Cohen et al.
Conflict of interest statement
Funded in part by the Joan and Irwin Jacobs Fund and by the National Cancer Institute grant P30 CA016672 (RK).
Figures

References
-
- Salivary duct carcinoma: targeting the phosphatidylinositol 3-kinase pathway by blocking mammalian target of rapamycin with temsirolimus. Piha-Paul SA, Cohen PR, Kurzrock R. J Clin Oncol. 2011;29:727–730. - PubMed
-
- The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. Cohen PR, Prieto VG, Piha-Paul SA, Kurzrock R. https://www.ncbi.nlm.nih.gov/pubmed/23050032 J Clin Aesthet Dermatol. 2012;5:27–36. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
