Therapeutic Opportunities of Targeting Allosteric Binding Sites on the Calcium-Sensing Receptor
- PMID: 33860192
- PMCID: PMC8033781
- DOI: 10.1021/acsptsci.1c00046
Therapeutic Opportunities of Targeting Allosteric Binding Sites on the Calcium-Sensing Receptor
Abstract
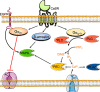
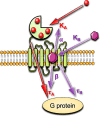
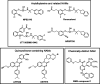
The CaSR is a class C G protein-coupled receptor (GPCR) that acts as a multimodal chemosensor to maintain diverse homeostatic functions. The CaSR is a clinical therapeutic target in hyperparathyroidism and has emerged as a putative target in several other diseases. These include hyper- and hypocalcaemia caused either by mutations in the CASR gene or in genes that regulate CaSR signaling and expression, and more recently in asthma. The development of CaSR-targeting drugs is complicated by the fact that the CaSR possesses many different binding sites for endogenous and exogenous agonists and allosteric modulators. Binding sites for endogenous and exogenous ligands are located throughout the large CaSR protein and are interconnected in ways that we do not yet fully understand. This review summarizes our current understanding of CaSR physiology, signaling, and structure and how the many different binding sites of the CaSR may be targeted to treat disease.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Glutathione-Based Photoaffinity Probe Identifies Caffeine as a Positive Allosteric Modulator of the Calcium-Sensing Receptor.ACS Chem Biol. 2024 Jul 19;19(7):1661-1670. doi: 10.1021/acschembio.4c00335. Epub 2024 Jul 8. ACS Chem Biol. 2024. PMID: 38975966 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function.Pharmacol Rev. 2020 Jul;72(3):558-604. doi: 10.1124/pr.119.018531. Pharmacol Rev. 2020. PMID: 32467152 Free PMC article. Review.
-
Molecular regulation of calcium-sensing receptor (CaSR)-mediated signaling.Chronic Dis Transl Med. 2024 Apr 29;10(3):167-194. doi: 10.1002/cdt3.123. eCollection 2024 Sep. Chronic Dis Transl Med. 2024. PMID: 39027195 Free PMC article. Review.
-
Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators.Endocrinology. 2013 Mar;154(3):1105-16. doi: 10.1210/en.2012-1887. Epub 2013 Jan 31. Endocrinology. 2013. PMID: 23372019
-
Development of AC265347-Inspired Calcium-Sensing Receptor Ago-Positive Allosteric Modulators.ChemMedChem. 2021 Nov 19;16(22):3451-3462. doi: 10.1002/cmdc.202100368. Epub 2021 Jul 29. ChemMedChem. 2021. PMID: 34216111
Cited by
-
Glutathione-Based Photoaffinity Probe Identifies Caffeine as a Positive Allosteric Modulator of the Calcium-Sensing Receptor.ACS Chem Biol. 2024 Jul 19;19(7):1661-1670. doi: 10.1021/acschembio.4c00335. Epub 2024 Jul 8. ACS Chem Biol. 2024. PMID: 38975966 Free PMC article.
-
Solid-phase extraction with the functionalization of calcium-sensing receptors onto magnetic microspheres as an affinity probe can capture ligands selectively from herbal extract.Mikrochim Acta. 2023 Dec 18;191(1):34. doi: 10.1007/s00604-023-06092-4. Mikrochim Acta. 2023. PMID: 38108923
-
Low molecular weight hyaluronan inhibits lung epithelial ion channels by activating the calcium-sensing receptor.Matrix Biol. 2023 Feb;116:67-84. doi: 10.1016/j.matbio.2023.02.002. Epub 2023 Feb 8. Matrix Biol. 2023. PMID: 36758905 Free PMC article.
-
Prolonged Diuretic, Natriuretic, and Potassium- and Calcium-Sparing Effect of Hesperidin in Hypertensive Rats.Plants (Basel). 2025 Apr 27;14(9):1324. doi: 10.3390/plants14091324. Plants (Basel). 2025. PMID: 40364353 Free PMC article.
-
Silicon-Rhodamine Functionalized Evocalcet Probes Potently and Selectively Label Calcium Sensing Receptors In Vitro, In Vivo, and Ex Vivo.ACS Pharmacol Transl Sci. 2024 Apr 25;7(5):1557-1570. doi: 10.1021/acsptsci.4c00096. eCollection 2024 May 10. ACS Pharmacol Transl Sci. 2024. PMID: 38751613 Free PMC article.
References
-
- Leach K.; Hannan F. M.; Josephs T. M.; Keller A. N.; Møller T. C.; Ward D. T.; Kallay E.; Mason R. S.; Thakker R. V.; Riccardi D.; Conigrave A. D.; Bräuner-Osborne H. (2020) International Union of Basic and Clinical Pharmacology. CVIII. Calcium-sensing receptor nomenclature, pharmacology, and function. Pharmacol. Rev. 72, 558–604. 10.1124/pr.119.018531. - DOI - PMC - PubMed
-
- Kantham L.; Quinn S. J.; Egbuna O. I.; Baxi K.; Butters R.; Pang J. L.; Pollak M. R.; Goltzman D.; Brown E. M. (2009) The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol. 297, E915–923. 10.1152/ajpendo.00315.2009. - DOI - PMC - PubMed
-
- Dvorak M. M.; Siddiqua A.; Ward D. T.; Carter D. H.; Dallas S. L.; Nemeth E. F.; Riccardi D. (2004) Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. U. S. A. 101, 5140–5145. 10.1073/pnas.0306141101. - DOI - PMC - PubMed
-
- Diepenhorst N. A.; Leach K.; Keller A. N.; Rueda P.; Cook A. E.; Pierce T. L.; Nowell C.; Pastoureau P.; Sabatini M.; Summers R. J.; Charman W. N.; Sexton P. M.; Christopoulos A.; Langmead C. J. (2018) Divergent effects of strontium and calcium-sensing receptor positive allosteric modulators (calcimimetics) on human osteoclast activity. Br. J. Pharmacol. 175, 4095–4108. 10.1111/bph.14344. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
