Gut microbial alterations in neonatal jaundice pre- and post-treatment
- PMID: 33860293
- PMCID: PMC8150162
- DOI: 10.1042/BSR20210362
Gut microbial alterations in neonatal jaundice pre- and post-treatment
Abstract
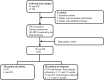
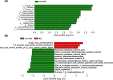
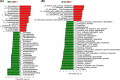
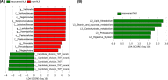
Neonatal jaundice is a common disease that affects up to 60% of newborns. Herein, we performed a comparative analysis of the gut microbiome in neonatal jaundice and non-neonatal jaundice infants (NJIs) and identified gut microbial alterations in neonatal jaundice pre- and post-treatment. We prospectively collected 232 fecal samples from 51 infants at five time points (0, 1, 3, 6, and 12 months). Finally, 114 samples from 6 NJIs and 19 non-NJI completed MiSeq sequencing and analysis. We characterized the gut microbiome and identified microbial differences and gene functions. Meconium microbial diversity from NJI was decreased compared with that from non-NJI. The genus Gemella was decreased in NJI versus non-NJI. Eleven predicted microbial functions, including fructose 1,6-bisphosphatase III and pyruvate carboxylase subunit B, decreased, while three functions, including acetyl-CoA acyltransferase, increased in NJI. After treatments, the microbial community presented significant alteration-based β diversity. The phyla Firmicutes and Actinobacteria were increased, while Proteobacteria and Fusobacteria were decreased. Microbial alterations were also analyzed between 6 recovered NJI and 19 non-NJI. The gut microbiota was unique in the meconium microbiome from NJI, implying that early gut microbiome intervention could be promising for the management of neonatal jaundice. Alterations of gut microbiota from NJI can be of great value to bolster evidence-based prevention against 'bacterial dysbiosis'.
Keywords: Gut microbiota; MiSeq sequencing; Neonatal jaundice; Treatment.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



Similar articles
-
Meconium microbiome associates with the development of neonatal jaundice.Clin Transl Gastroenterol. 2018 Sep 20;9(9):182. doi: 10.1038/s41424-018-0048-x. Clin Transl Gastroenterol. 2018. PMID: 30237489 Free PMC article.
-
Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis.J Nutr Biochem. 2019 Jan;63:54-61. doi: 10.1016/j.jnutbio.2018.09.016. Epub 2018 Sep 26. J Nutr Biochem. 2019. PMID: 30342317
-
Gut microbiome dysbiosis as a potential biomarker for liver metabolic disorders in in neonatal hemolytic jaundice.BMC Pediatr. 2025 Apr 29;25(1):337. doi: 10.1186/s12887-025-05692-8. BMC Pediatr. 2025. PMID: 40301849 Free PMC article.
-
What Happens in the Gut during the Formation of Neonatal Jaundice-Underhand Manipulation of Gut Microbiota?Int J Mol Sci. 2024 Aug 6;25(16):8582. doi: 10.3390/ijms25168582. Int J Mol Sci. 2024. PMID: 39201270 Free PMC article. Review.
-
The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease.Front Vet Sci. 2020 Jan 14;6:498. doi: 10.3389/fvets.2019.00498. eCollection 2019. Front Vet Sci. 2020. PMID: 31993446 Free PMC article. Review.
Cited by
-
The correlation between serum total bile acid and alanine aminotransferase of pregnant women and the disorders of neonatal hyperbilirubinemia-related amino acid metabolism.BMC Pregnancy Childbirth. 2024 Jan 3;24(1):26. doi: 10.1186/s12884-023-06226-9. BMC Pregnancy Childbirth. 2024. PMID: 38172739 Free PMC article.
-
Analysis of the intestinal microbiota and profiles of blood amino acids and acylcarnitines in neonates with hyperbilirubinemia.BMC Microbiol. 2024 May 18;24(1):171. doi: 10.1186/s12866-024-03328-y. BMC Microbiol. 2024. PMID: 38760685 Free PMC article.
-
Investigating prenatal and perinatal factors on meconium microbiota: a systematic review and cohort study.Pediatr Res. 2024 Jan;95(1):135-145. doi: 10.1038/s41390-023-02783-z. Epub 2023 Aug 17. Pediatr Res. 2024. PMID: 37591927 Free PMC article.
-
Association of breast milk microbiota and metabolites with neonatal jaundice.Front Pediatr. 2025 Jan 6;12:1500069. doi: 10.3389/fped.2024.1500069. eCollection 2024. Front Pediatr. 2025. PMID: 39834492 Free PMC article.
-
Temporal Investigation of the Maternal Origins of Fetal Gut Microbiota.Microorganisms. 2024 Sep 9;12(9):1865. doi: 10.3390/microorganisms12091865. Microorganisms. 2024. PMID: 39338539 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

