Fibroblast Growth Factor Signalling in the Diseased Nervous System
- PMID: 33860438
- PMCID: PMC8280051
- DOI: 10.1007/s12035-021-02367-0
Fibroblast Growth Factor Signalling in the Diseased Nervous System
Abstract
Fibroblast growth factors (FGFs) act as key signalling molecules in brain development, maintenance, and repair. They influence the intricate relationship between myelinating cells and axons as well as the association of astrocytic and microglial processes with neuronal perikarya and synapses. Advances in molecular genetics and imaging techniques have allowed novel insights into FGF signalling in recent years. Conditional mouse mutants have revealed the functional significance of neuronal and glial FGF receptors, not only in tissue protection, axon regeneration, and glial proliferation but also in instant behavioural changes. This review provides a summary of recent findings regarding the role of FGFs and their receptors in the nervous system and in the pathogenesis of major neurological and psychiatric disorders.
Keywords: Anxiety; Brain; CNS; Degeneration; Demyelination; Depression; FGF; FGFR; Ganglia; Glioma; Nerve; PNS; Receptor; Regeneration; Remyelination; Schizophrenia; Spinal cord.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
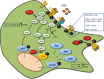
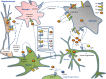
Similar articles
-
Roles of Fibroblast Growth Factors in the Axon Guidance.Int J Mol Sci. 2023 Jun 18;24(12):10292. doi: 10.3390/ijms241210292. Int J Mol Sci. 2023. PMID: 37373438 Free PMC article. Review.
-
FGF/FGFR signaling in bone formation: progress and perspectives.Growth Factors. 2012 Apr;30(2):117-23. doi: 10.3109/08977194.2012.656761. Epub 2012 Feb 1. Growth Factors. 2012. PMID: 22292523 Review.
-
The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration.Cells. 2021 Nov 19;10(11):3242. doi: 10.3390/cells10113242. Cells. 2021. PMID: 34831463 Free PMC article. Review.
-
Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders.Semin Cell Dev Biol. 2016 May;53:136-43. doi: 10.1016/j.semcdb.2015.10.003. Epub 2015 Oct 23. Semin Cell Dev Biol. 2016. PMID: 26454097 Free PMC article. Review.
-
Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease.J Biochem. 2011 Feb;149(2):121-30. doi: 10.1093/jb/mvq121. Epub 2010 Oct 12. J Biochem. 2011. PMID: 20940169 Free PMC article. Review.
Cited by
-
Roles of Fibroblast Growth Factors in the Axon Guidance.Int J Mol Sci. 2023 Jun 18;24(12):10292. doi: 10.3390/ijms241210292. Int J Mol Sci. 2023. PMID: 37373438 Free PMC article. Review.
-
FGF21, a modulator of astrocyte reactivity, protects against ischemic brain injury through anti-inflammatory and neurotrophic pathways.Acta Pharmacol Sin. 2025 Jul;46(7):1834-1851. doi: 10.1038/s41401-024-01462-x. Epub 2025 Feb 28. Acta Pharmacol Sin. 2025. PMID: 40021824 Free PMC article.
-
Growth Factors and Their Application in the Therapy of Hereditary Neurodegenerative Diseases.Biomedicines. 2024 Aug 20;12(8):1906. doi: 10.3390/biomedicines12081906. Biomedicines. 2024. PMID: 39200370 Free PMC article. Review.
-
FGF/FGFR system in the central nervous system demyelinating disease: Recent progress and implications for multiple sclerosis.CNS Neurosci Ther. 2023 Jun;29(6):1497-1511. doi: 10.1111/cns.14176. Epub 2023 Mar 16. CNS Neurosci Ther. 2023. PMID: 36924298 Free PMC article. Review.
-
c-Cbl Regulates Murine Subventricular Zone-Derived Neural Progenitor Cells in Dependence of the Epidermal Growth Factor Receptor.Cells. 2023 Oct 3;12(19):2400. doi: 10.3390/cells12192400. Cells. 2023. PMID: 37830613 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

