Multifunctional Heterometallic IrIII -AuI Probes as Promising Anticancer and Antiangiogenic Agents
- PMID: 33860585
- PMCID: PMC8361937
- DOI: 10.1002/chem.202100707
Multifunctional Heterometallic IrIII -AuI Probes as Promising Anticancer and Antiangiogenic Agents
Abstract
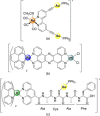
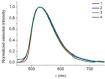
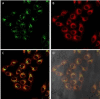
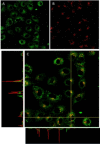
A new class of emissive cyclometallated IrIII -AuI complexes with a bis(diphenylphosphino) methanide bridging ligand was successfully synthesised from the diphosphino complex [Ir(N^C)2 (dppm)]+ (1). The different gold ancillary ligand, a triphenylphosphine (2), a chloride (3) or a thiocytosine (4) did not reveal any significant effect on the photophysical properties, which are mainly due to metal-to-ligand charge-transfer (3 MLCT) transitions based on IrIII . However, the AuI fragment, along with the ancillary ligand, seemed crucial for the bioactivity in A549 lung carcinoma cells versus endothelial cells. Both cell types display variable sensitivities to the complexes (IC50 =0.6-3.5 μM). The apoptotic pathway is activated in all cases, and paraptotic cell death seems to take place at initial stages in A549 cells. Species 2-4 showed at least dual lysosomal and mitochondrial biodistribution in A549 cells, with an initial lysosomal localisation and a possible trafficking process between both organelles with time. The bimetallic IrIII -AuI complexes disrupted the mitochondrial transmembrane potential in A549 cells and increased reactive oxygen species (ROS) generation and thioredoxin reductase (TrxR) inhibition in comparison with that displayed by the monometallic complex 1. Angiogenic activity assays performed in endothelial cells revealed the promising antimetastatic potential of 1, 2 and 4.
Keywords: anti-angiogenesis; cell imaging; gold; heterometallic compounds; iridium; theranostic agents.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Luminescent Bimetallic IrIII /AuI Peptide Bioconjugates as Potential Theranostic Agents.Chemistry. 2020 Sep 21;26(53):12158-12167. doi: 10.1002/chem.202002067. Epub 2020 Sep 2. Chemistry. 2020. PMID: 32542887 Free PMC article.
-
Highly potent half-sandwich iridium and ruthenium complexes as lysosome-targeted imaging and anticancer agents.Dalton Trans. 2018 Nov 13;47(44):15772-15782. doi: 10.1039/c8dt02963f. Dalton Trans. 2018. PMID: 30357192
-
Thiabendazole-based Rh(III) and Ir(III) biscyclometallated complexes with mitochondria-targeted anticancer activity and metal-sensitive photodynamic activity.Eur J Med Chem. 2018 Sep 5;157:279-293. doi: 10.1016/j.ejmech.2018.07.065. Epub 2018 Aug 2. Eur J Med Chem. 2018. PMID: 30099251
-
Novel and Versatile Imine-N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes as Lysosome-Targeted Anticancer Agents.Inorg Chem. 2018 Sep 4;57(17):11087-11098. doi: 10.1021/acs.inorgchem.8b01656. Epub 2018 Aug 22. Inorg Chem. 2018. PMID: 30133276
-
Chemistry, structure, and biological roles of Au-NHC complexes as TrxR inhibitors.Bioorg Chem. 2020 Jan;95:103552. doi: 10.1016/j.bioorg.2019.103552. Epub 2019 Dec 24. Bioorg Chem. 2020. PMID: 31911299 Review.
Cited by
-
Synthesis and Antiproliferative Insights of Lipophilic Ru(II)-Hydroxy Stearic Acid Hybrid Species.Molecules. 2023 May 12;28(10):4051. doi: 10.3390/molecules28104051. Molecules. 2023. PMID: 37241793 Free PMC article.
-
Dual Emissive Ir(III) Complexes for Photodynamic Therapy and Bioimaging.Pharmaceutics. 2021 Sep 1;13(9):1382. doi: 10.3390/pharmaceutics13091382. Pharmaceutics. 2021. PMID: 34575458 Free PMC article.
-
Promising heterometallic compounds as anticancer agents: Recent studies in vivo.Curr Opin Chem Biol. 2023 Feb;72:102250. doi: 10.1016/j.cbpa.2022.102250. Epub 2022 Dec 23. Curr Opin Chem Biol. 2023. PMID: 36566618 Free PMC article. Review.
-
Tunable Emissive Ir(III) Benzimidazole-quinoline Hybrids as Promising Theranostic Lead Compounds.ChemMedChem. 2022 Sep 16;17(18):e202200244. doi: 10.1002/cmdc.202200244. Epub 2022 Jul 13. ChemMedChem. 2022. PMID: 35767349 Free PMC article.
-
Highlights of New Strategies to Increase the Efficacy of Transition Metal Complexes for Cancer Treatments.Molecules. 2022 Dec 29;28(1):273. doi: 10.3390/molecules28010273. Molecules. 2022. PMID: 36615466 Free PMC article. Review.
References
-
- Barnard C., Johnson Matthey Technol. Rev. 2017, 61, 52–59.
-
- Oun R., Moussa Y. E., Wheate N. J., Dalton Trans. 2018, 47, 6645–6653. - PubMed
-
- Fernández-Moreira V., Herrera R. P., Gimeno M. C., Pure Appl. Chem. 2019, 91, 247–269.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

