Intracerebroventricular Administration of AAV9-PHP.B SYN1-EmGFP Induces Widespread Transgene Expression in the Mouse and Monkey Central Nervous System
- PMID: 33860682
- PMCID: PMC8236560
- DOI: 10.1089/hum.2020.301
Intracerebroventricular Administration of AAV9-PHP.B SYN1-EmGFP Induces Widespread Transgene Expression in the Mouse and Monkey Central Nervous System
Abstract
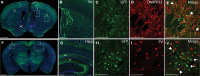
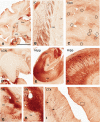
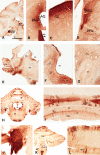
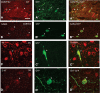
Viral vectors made from adeno-associated virus (AAV) have emerged as preferred tools in basic and translational neuroscience research to introduce or modify genetic material in cells of interest. The use of viral vectors is particularly attractive in nontransgenic species, such as nonhuman primates. Injection of AAV solutions into the cerebrospinal fluid is an effective method to achieve a broad distribution of a transgene in the central nervous system. In this study, we conducted injections of AAV9-PHP.B, a recently described AAV capsid mutant, in the lateral ventricle of mice and rhesus macaques. To enhance the expression of the transgene (the tag protein emerald green fluorescent protein [EmGFP]), we used a gene promoter that confers high neuron-specific expression of the transgene, the human synapsin 1 (SYN1) promoter. The efficacy of the viral vector was first tested in mice. Our results show that intracerebroventricular injections of AAV9-PHP.B SYN1-EmGFP-woodchuck hepatitis virus posttranscriptional regulatory element resulted in neuronal EmGFP expression throughout the mice and monkey brains. We have provided a thorough characterization of the brain regions expressing EmGFP in both species. EmGFP was observed in neuronal cell bodies over the whole cerebral cortex and in the cerebellum, as well as in some subcortical regions, including the striatum and hippocampus. We also observed densely labeled neuropil in areas known to receive projections from these regions. Double fluorescence studies demonstrated that EmGFP was expressed by several types of neurons throughout the mouse and monkey brain. Our results demonstrate that a single injection in the lateral ventricle is an efficient method to obtain transgene expression in many cortical and subcortical regions, obviating the need of multiple intraparenchymal injections to cover large brain areas. The use of intraventricular injections of AAV9-PHP.B SYN1-EmGFP could provide a powerful approach to transduce widespread areas of the brain and may contribute to further development of methods to genetically target-specific populations of neurons.
Keywords: ICV; adeno-associated virus; gene therapy; primate; synapsin.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Ojala DS, Amara DP, Schaffer DV. Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 2015;21:84–98 - PubMed
-
- Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. In: Friedmann T, ed. Gene Transfer Vectors for Clinical Application. Amsterdam, Netherlands: Elsevier/Academis Press, 2012:229–254 - PubMed
-
- Saraiva J, Nobre RJ, Pereira de Almeida L. Gene therapy for the CNS using AAVs: the impact of systemic delivery by AAV9. J Control Release 2016;241:94–109 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

