The Nesprin-1/-2 ortholog ANC-1 regulates organelle positioning in C. elegans independently from its KASH or actin-binding domains
- PMID: 33860766
- PMCID: PMC8139857
- DOI: 10.7554/eLife.61069
The Nesprin-1/-2 ortholog ANC-1 regulates organelle positioning in C. elegans independently from its KASH or actin-binding domains
Abstract
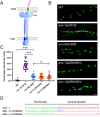
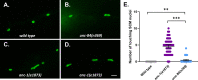
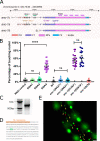
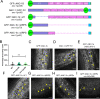
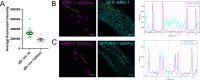
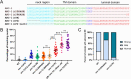
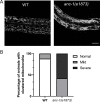
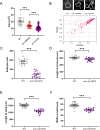
KASH proteins in the outer nuclear membrane comprise the cytoplasmic half of linker of nucleoskeleton and cytoskeleton (LINC) complexes that connect nuclei to the cytoskeleton. Caenorhabditis elegans ANC-1, an ortholog of Nesprin-1/2, contains actin-binding and KASH domains at opposite ends of a long spectrin-like region. Deletion of either the KASH or calponin homology (CH) domains does not completely disrupt nuclear positioning, suggesting neither KASH nor CH domains are essential. Deletions in the spectrin-like region of ANC-1 led to significant defects, but only recapitulated the null phenotype in combination with mutations in the transmembrane (TM) span. In anc-1 mutants, the endoplasmic reticulum ER, mitochondria, and lipid droplets were unanchored, moving throughout the cytoplasm. The data presented here support a cytoplasmic integrity model where ANC-1 localizes to the ER membrane and extends into the cytoplasm to position nuclei, ER, mitochondria, and other organelles in place.
Keywords: C. elegans; ER; LINC complexes; cell biology; nesprin; nuclear envelope; nuclear positioning.
© 2021, Hao et al.
Conflict of interest statement
HH, SK, LJ, LG, NC, JB, DS No competing interests declared
Figures







Comment in
-
Putting organelles in their place.Elife. 2021 May 21;10:e69422. doi: 10.7554/eLife.69422. Elife. 2021. PMID: 34018487 Free PMC article.
Similar articles
-
Role of ANC-1 in tethering nuclei to the actin cytoskeleton.Science. 2002 Oct 11;298(5592):406-9. doi: 10.1126/science.1075119. Epub 2002 Aug 8. Science. 2002. PMID: 12169658
-
Conserved SUN-KASH Interfaces Mediate LINC Complex-Dependent Nuclear Movement and Positioning.Curr Biol. 2018 Oct 8;28(19):3086-3097.e4. doi: 10.1016/j.cub.2018.08.001. Epub 2018 Sep 20. Curr Biol. 2018. PMID: 30245107 Free PMC article.
-
Reinforcing the LINC complex connection to actin filaments: the role of FHOD1 in TAN line formation and nuclear movement.Cell Cycle. 2015;14(14):2200-5. doi: 10.1080/15384101.2015.1053665. Epub 2015 Jun 17. Cell Cycle. 2015. PMID: 26083340 Free PMC article.
-
SUN/KASH interactions facilitate force transmission across the nuclear envelope.Nucleus. 2019 Dec;10(1):73-80. doi: 10.1080/19491034.2019.1595313. Nucleus. 2019. PMID: 30888237 Free PMC article. Review.
-
KASHing up with the nucleus: novel functional roles of KASH proteins at the cytoplasmic surface of the nucleus.Curr Opin Cell Biol. 2014 Jun;28:69-75. doi: 10.1016/j.ceb.2014.03.002. Epub 2014 Apr 3. Curr Opin Cell Biol. 2014. PMID: 24704701 Free PMC article. Review.
Cited by
-
Putting organelles in their place.Elife. 2021 May 21;10:e69422. doi: 10.7554/eLife.69422. Elife. 2021. PMID: 34018487 Free PMC article.
-
Nuclear movement in multinucleated cells.Development. 2022 Nov 1;149(21):dev200749. doi: 10.1242/dev.200749. Epub 2022 Oct 28. Development. 2022. PMID: 36305464 Free PMC article. Review.
-
Mechanics and functional consequences of nuclear deformations.Nat Rev Mol Cell Biol. 2022 Sep;23(9):583-602. doi: 10.1038/s41580-022-00480-z. Epub 2022 May 5. Nat Rev Mol Cell Biol. 2022. PMID: 35513718 Free PMC article. Review.
-
Katanin, kinesin-13 and ataxin-2 inhibit premature interaction between maternal and paternal genomes in C. elegans zygotes.bioRxiv [Preprint]. 2024 Jun 26:2024.03.12.584242. doi: 10.1101/2024.03.12.584242. bioRxiv. 2024. Update in: Elife. 2024 Jul 30;13:RP97812. doi: 10.7554/eLife.97812. PMID: 38559153 Free PMC article. Updated. Preprint.
-
A distinct isoform of Msp300 (nesprin) organizes the perinuclear microtubule organizing center in adipocytes.bioRxiv [Preprint]. 2024 Dec 16:2024.06.28.601268. doi: 10.1101/2024.06.28.601268. bioRxiv. 2024. Update in: Mol Biol Cell. 2025 Jul 1;36(7):ar92. doi: 10.1091/mbc.E25-01-0003. PMID: 38979285 Free PMC article. Updated. Preprint.
References
-
- Altun Z, Hall D. Epithelial System, Hypodermis. WormAtlas; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

