Transcription factors regulated by cAMP in smooth muscle of the myometrium at human parturition
- PMID: 33860781
- PMCID: PMC8106496
- DOI: 10.1042/BST20201173
Transcription factors regulated by cAMP in smooth muscle of the myometrium at human parturition
Abstract
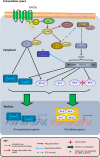
Cyclic adenosine monophosphate (cAMP) contributes to maintenance of a quiescent (relaxed) state in the myometrium (i.e. uterine smooth muscle) during pregnancy, which most commonly has been attributed to activation of protein kinase A (PKA). PKA-mediated phosphorylation of cytosolic contractile apparatus components in myometrial smooth muscle cells (mSMCs) are known to promote relaxation. Additionally, PKA also regulates nuclear transcription factor (TF) activity to control expression of genes important to the labour process; these are mostly involved in actin-myosin interactions, cell-to-cell connectivity and inflammation, all of which influence mSMC transition from a quiescent to a contractile (pro-labour) phenotype. This review focuses on the evidence that cAMP modulates the activity of TFs linked to pro-labour gene expression, predominantly cAMP response element (CRE) binding TFs, nuclear factor κB (NF-κB), activator protein 1 (AP-1) family and progesterone receptors (PRs). This review also considers the more recently described exchange protein directly activated by cAMP (EPAC) that may oppose the pro-quiescent effects of PKA, as well as explores findings from other cell types that have the potential to be of novel relevance to cAMP action on TF function in the myometrium.
Keywords: bZIP transcription factors; cAMP; muscle contraction; pregnancy; signal transducers and activators of transcription; uterus.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Cyclic AMP Effectors Regulate Myometrial Oxytocin Receptor Expression.Endocrinology. 2016 Nov;157(11):4411-4422. doi: 10.1210/en.2016-1514. Epub 2016 Sep 27. Endocrinology. 2016. PMID: 27673556
-
Characterization and functional analysis of cAMP response element modulator protein and activating transcription factor 2 (ATF2) isoforms in the human myometrium during pregnancy and labor: identification of a novel ATF2 species with potent transactivation properties.J Clin Endocrinol Metab. 2002 Apr;87(4):1717-28. doi: 10.1210/jcem.87.4.8360. J Clin Endocrinol Metab. 2002. PMID: 11932306
-
Differential impact of acute and prolonged cAMP agonist exposure on protein kinase A activation and human myometrium contractile activity.J Physiol. 2016 Nov 1;594(21):6369-6393. doi: 10.1113/JP272320. Epub 2016 Aug 8. J Physiol. 2016. PMID: 27328735 Free PMC article.
-
Transcriptional control of parturition: insights from gene regulation studies in the myometrium.Mol Hum Reprod. 2021 May 8;27(5):gaab024. doi: 10.1093/molehr/gaab024. Mol Hum Reprod. 2021. PMID: 33823545 Free PMC article. Review.
-
NF-kappaB function in the human myometrium during pregnancy and parturition.Histol Histopathol. 2010 Jul;25(7):945-56. doi: 10.14670/HH-25.945. Histol Histopathol. 2010. PMID: 20503182 Review.
Cited by
-
cAMP Compartmentalisation in Human Myometrial Cells.Cells. 2023 Feb 24;12(5):718. doi: 10.3390/cells12050718. Cells. 2023. PMID: 36899855 Free PMC article.
-
ATF2 loss promotes 5-FU resistance in colon cancer cells via activation of the ATR-Chk1 damage response pathway.BMC Cancer. 2023 May 27;23(1):480. doi: 10.1186/s12885-023-10940-0. BMC Cancer. 2023. PMID: 37237279 Free PMC article.
-
Regulation of 20α-Hydroxysteroid Dehydrogenase Expression in Term Pregnant Human Myometrium Ex Vivo.Reprod Sci. 2024 Jan;31(1):150-161. doi: 10.1007/s43032-023-01333-6. Epub 2023 Aug 30. Reprod Sci. 2024. PMID: 37648943 Free PMC article.
-
Estrogen receptors in mitochondrial metabolism: age-related changes and implications for pregnancy complications.Aging Adv. 2024 Dec;1(2):154-171. doi: 10.4103/agingadv.agingadv-d-24-00012. Epub 2024 Dec 20. Aging Adv. 2024. PMID: 39839811 Free PMC article.
-
Integrated Proteotranscriptomics of Human Myometrium in Labor Landscape Reveals the Increased Molecular Associated With Inflammation Under Hypoxia Stress.Front Immunol. 2021 Oct 4;12:722816. doi: 10.3389/fimmu.2021.722816. eCollection 2021. Front Immunol. 2021. PMID: 34671346 Free PMC article.
References
-
- Taggart, M.J. and Tribe, R.M. (2007) Cellular ionic mechanisms controlling uterine smooth muscle contraction: effects of gestational state. In New Frontiers in Smooth Muscle Biology and Physiology (Savinneau, J., ed.), pp. 523–549, Research Signpost, India
-
- Romero, R., Miranda, J., Chaiworapongsa, T., Korzeniewski, S.J., Chaemsaithong, P., Gotsch, F.et al. (2014) Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol. 72, 458–474 10.1111/aji.12296 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

