The control of polycomb repressive complexes by long noncoding RNAs
- PMID: 33861025
- PMCID: PMC8500928
- DOI: 10.1002/wrna.1657
The control of polycomb repressive complexes by long noncoding RNAs
Abstract
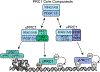
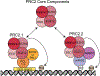
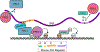
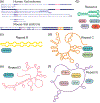
The polycomb repressive complexes 1 and 2 (PRCs; PRC1 and PRC2) are conserved histone-modifying enzymes that often function cooperatively to repress gene expression. The PRCs are regulated by long noncoding RNAs (lncRNAs) in complex ways. On the one hand, specific lncRNAs cause the PRCs to engage with chromatin and repress gene expression over genomic regions that can span megabases. On the other hand, the PRCs bind RNA with seemingly little sequence specificity, and at least in the case of PRC2, direct RNA-binding has the effect of inhibiting the enzyme. Thus, some RNAs appear to promote PRC activity, while others may inhibit it. The reasons behind this apparent dichotomy are unclear. The most potent PRC-activating lncRNAs associate with chromatin and are predominantly unspliced or harbor unusually long exons. Emerging data imply that these lncRNAs promote PRC activity through internal RNA sequence elements that arise and disappear rapidly in evolutionary time. These sequence elements may function by interacting with common subsets of RNA-binding proteins that recruit or stabilize PRCs on chromatin. This article is categorized under: RNA Interactions with Proteins and Other Molecules > Protein-RNA Recognition RNA Interactions with Proteins and Other Molecules > RNA-Protein Complexes RNA Interactions with Proteins and Other Molecules > Protein-RNA Interactions: Functional Implications.
Keywords: Airn; HOTAIR; Xist; lncRNA; polycomb.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Figures


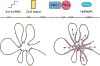
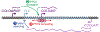
References
-
- Almeida M, Pintacuda G, Masui O, Koseki Y, Gdula M, Cerase A, Brown D, Mould A, Innocent C, Nakayama M, Schermelleh L, Nesterova TB, Koseki H, & Brockdorff N (2017). PCGF3/5-PRC1 initiates polycomb recruitment in X chromosome inactivation. Science, 356(6342), 1081–1084. 10.1126/science.aal2512 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

