Clinical characteristics and antibody response to SARS-CoV-2 spike 1 protein using VITROS Anti-SARS-CoV-2 antibody tests in COVID-19 patients in Japan
- PMID: 33861191
- PMCID: PMC8289209
- DOI: 10.1099/jmm.0.001291
Clinical characteristics and antibody response to SARS-CoV-2 spike 1 protein using VITROS Anti-SARS-CoV-2 antibody tests in COVID-19 patients in Japan
Abstract
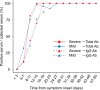
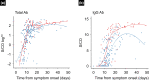
Introduction. Serological tests for COVID-19 are important in providing results for surveillance and supporting diagnosis. Investigating the serological response in COVID-19 patients with different disease severity is important for assessing the clinical utility of serological assays.Gap Statement. However, few studies have investigated the clinical utility of antibody assays for COVID-19 or differences in antibody response in association with disease severity.Aim. The study aimed to evaluate the clinical characteristics and clinical utility of VITROS SARS-CoV-2 antibody tests according to COVID-19 severity in patients in Japan.Methodology. We analysed 255 serum specimens from 130 COVID-19 patients and examined clinical records and laboratory data. Presence of total (IgA, IgM, and IgG) and specific IgG antibody for the spike 1 antigen of SARS-CoV-2 was determined using VITROS Anti-SARS-CoV-2 antibody tests.Results. Overall, 98 (75.4 %) and 32 (24.6 %) patients had mild and severe COVID-19, respectively. On admission, 76 (58.5 %) and 45 (34.6 %) patients were positive for total and IgG antibody assays. Among 91 patients at discharge, 90 (98.9 %) and 81 (89.0 %) were positive for total and IgG antibody, respectively. Clinical background and laboratory findings on admission, but not the prevalence or concentration of total or IgG antibody, were associated with disease prognosis. Total and IgG antibody intensities were significantly higher in severe cases than in mild cases in serum collected >11 days after onset, but not within 10 days.Conclusion. VITROS Anti-SARS-CoV-2 total and IgG assays will be useful as supporting diagnostic and surveillance tools and for evaluation of humoral immune response to COVID-19. Optimal prediction of disease prognosis is made from considering both clinical history and laboratory findings.
Keywords: COVID-19; Japan; SARS-CoV-2; antibody; chemiluminescent immunoassay.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Validation of a new automated chemiluminescent anti-SARS-CoV-2 IgM and IgG antibody assay system detecting both N and S proteins in Japan.PLoS One. 2021 Mar 4;16(3):e0247711. doi: 10.1371/journal.pone.0247711. eCollection 2021. PLoS One. 2021. PMID: 33661990 Free PMC article.
-
Inference of SARS-CoV-2 spike-binding neutralizing antibody titers in sera from hospitalized COVID-19 patients by using commercial enzyme and chemiluminescent immunoassays.Eur J Clin Microbiol Infect Dis. 2021 Mar;40(3):485-494. doi: 10.1007/s10096-020-04128-8. Epub 2021 Jan 6. Eur J Clin Microbiol Infect Dis. 2021. PMID: 33404891 Free PMC article.
-
Diagnostic performance of four SARS-CoV-2 antibody assays in patients with COVID-19 or with bacterial and non-SARS-CoV-2 viral respiratory infections.Eur J Clin Microbiol Infect Dis. 2021 Sep;40(9):1983-1997. doi: 10.1007/s10096-021-04285-4. Epub 2021 Jun 9. Eur J Clin Microbiol Infect Dis. 2021. PMID: 34109500 Free PMC article.
-
Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review.Int J Infect Dis. 2021 Mar;104:415-422. doi: 10.1016/j.ijid.2021.01.016. Epub 2021 Jan 12. Int J Infect Dis. 2021. PMID: 33450372 Free PMC article.
-
Humoral immunological kinetics of severe acute respiratory syndrome coronavirus 2 infection and diagnostic performance of serological assays for coronavirus disease 2019: an analysis of global reports.Int Health. 2022 Jan 19;14(1):18-52. doi: 10.1093/inthealth/ihab005. Int Health. 2022. PMID: 33620427 Free PMC article. Review.
Cited by
-
Determinants of health as predictors for differential antibody responses following SARS-CoV-2 primary and booster vaccination in an at-risk, longitudinal cohort.PLoS One. 2024 Apr 2;19(4):e0292566. doi: 10.1371/journal.pone.0292566. eCollection 2024. PLoS One. 2024. PMID: 38564600 Free PMC article.
-
Response kinetics of different classes of antibodies to SARS-CoV2 infection in the Japanese population: The IgA and IgG titers increased earlier than the IgM titers.Int Immunopharmacol. 2022 Feb;103:108491. doi: 10.1016/j.intimp.2021.108491. Epub 2021 Dec 21. Int Immunopharmacol. 2022. PMID: 34954559 Free PMC article.
-
Predictors for reactogenicity and humoral immunity to SARS-CoV-2 following infection and mRNA vaccination: A regularized, mixed-effects modelling approach.Front Immunol. 2023 Feb 9;14:971277. doi: 10.3389/fimmu.2023.971277. eCollection 2023. Front Immunol. 2023. PMID: 36845120 Free PMC article.
-
Progression and Predictors of SARS-CoV-2 Antibody Seroreactivity In US Blood Donors.Transfus Med Rev. 2021 Jul;35(3):8-15. doi: 10.1016/j.tmrv.2021.07.003. Epub 2021 Jul 30. Transfus Med Rev. 2021. PMID: 34376289 Free PMC article.
References
-
- World Health Organization [Jul 14;2020 ]; https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

