Usability of a Mobile App for Real-Time Assessment of Fatigue and Related Symptoms in Patients With Multiple Sclerosis: Observational Study
- PMID: 33861208
- PMCID: PMC8087974
- DOI: 10.2196/19564
Usability of a Mobile App for Real-Time Assessment of Fatigue and Related Symptoms in Patients With Multiple Sclerosis: Observational Study
Abstract
Background: Although fatigue is one of the most debilitating symptoms in patients with multiple sclerosis (MS), its pathogenesis is not well understood. Neurogenic, inflammatory, endocrine, and metabolic mechanisms have been proposed. Taking into account the temporal dynamics and comorbid mood symptoms of fatigue may help differentiate fatigue phenotypes. These phenotypes may reflect different pathogeneses and may respond to different mechanism-specific treatments. Although several tools have been developed to assess various symptoms (including fatigue), monitor clinical status, or improve the perceived level of fatigue in patients with MS, options for a detailed, real-time assessment of MS-related fatigue and relevant comorbidities are still limited.
Objective: This study aims to present a novel mobile app specifically designed to differentiate fatigue phenotypes using circadian symptom monitoring and state-of-the-art characterization of MS-related fatigue and its related symptoms. We also aim to report the first findings regarding patient compliance and the relationship between compliance and patient characteristics, including MS disease severity.
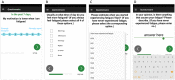
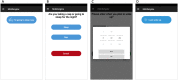
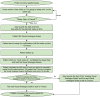
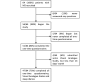
Methods: After developing the app, we used it in a prospective study designed to investigate the brain magnetic resonance imaging correlates of MS-related fatigue. In total, 64 patients with MS were recruited into this study and asked to use the app over a 2-week period. The app features the following modules: Visual Analogue Scales (VASs) to assess circadian changes in fatigue, depression, anxiety, and pain; daily sleep diaries (SLDs) to assess sleep habits and quality; and 10 one-time questionnaires to assess fatigue, depression, anxiety, sleepiness, physical activity, and motivation, as well as several other one-time questionnaires that were created to assess those relevant aspects of fatigue that were not captured by existing fatigue questionnaires. The app prompts subjects to assess their symptoms multiple times a day and enables real-time symptom monitoring through a web-accessible portal.
Results: Of 64 patients, 56 (88%) used the app, of which 51 (91%) completed all one-time questionnaires and 47 (84%) completed all one-time questionnaires, VASs, and SLDs. Patients reported no issues with the usage of the app, and there were no technical issues with our web-based data collection system. The relapsing-remitting MS to secondary-progressive MS ratio was significantly higher in patients who completed all one-time questionnaires, VASs, and SLDs than in those who completed all one-time questionnaires but not all VASs and SLDs (P=.01). No other significant differences in demographics, fatigue, or disease severity were observed between the degrees of compliance.
Conclusions: The app can be used with reasonable compliance across patients with relapsing-remitting and secondary-progressive MS irrespective of demographics, fatigue, or disease severity.
Keywords: depression; fatigue; mobile application; mobile phone; multiple sclerosis; real-time assessment.
©Miklos Palotai, Max Wallack, Gergo Kujbus, Adam Dalnoki, Charles Guttmann. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 16.04.2021.
Conflict of interest statement
Conflicts of Interest: MP and MW reported no disclosures. GK is the director of the Mobilengine. AD is the CEO of the Mobilengine. CG has received support from Mobilengine (free use of platform and programming by Mobilengine Engineers), the National Multiple Sclerosis Society, the International Progressive Multiple Sclerosis Alliance, the US Office for Naval Research, and travel support from Roche Pharmaceuticals; CG owns stock in Roche, Novartis, GlaxoSmithKline, Alnylam, Protalix Biotherapeutics, Arrowhead Pharmaceuticals, Cocrystal Pharma, and Sangamo Therapeutics.
Figures


References
-
- Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, Cutter GR, Kaye WE, Wagner L, Tremlett H, Buka SL, Dilokthornsakul P, Topol B, Chen LH, LaRocca NG. The prevalence of MS in the United States. Neurology. 2019 Feb 15;92(10):1029–40. doi: 10.1212/wnl.0000000000007035. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

