Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia
- PMID: 33861303
- PMCID: PMC8061088
- DOI: 10.1182/blood.2021011568
Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia
Abstract
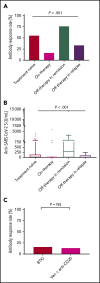
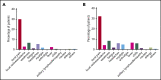
Patients with chronic lymphocytic leukemia (CLL) have an increased risk for severe COVID-19 disease and mortality. The goal of this study was to determine the efficacy of COVID-19 vaccine in patients with CLL. We evaluated humoral immune responses to the BNT162b2 messenger RNA (mRNA) COVID-19 vaccine in patients with CLL and compared responses with those obtained in age-matched healthy control subjects. Patients received 2 vaccine doses, 21 days apart, and antibody titers were measured by using the Elecsys Anti-SARS-CoV-2 S assay after administration of the second dose. In a total of 167 patients with CLL, the antibody response rate was 39.5%. A comparison between 52 patients with CLL and 52 sex- and aged-matched healthy control subjects revealed a significantly reduced response rate among patients (52% vs 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001). The response rate was highest in patients who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients and 16.0% in patients under treatment at the time of vaccination. In patients treated with either Bruton's tyrosine kinase inhibitors or venetoclax ± anti-CD20 antibody, response rates were considerably low (16.0% and 13.6%). None of the patients exposed to anti-CD20 antibodies <12 months before vaccination responded. In a multivariate analysis, the independent predictors of response were younger age, female sex, lack of currently active treatment, immunoglobulin G levels ≥550 mg/dL, and immunoglobulin M levels ≥40 mg/dL. In conclusion, antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in patients with CLL is markedly impaired and affected by disease activity and treatment. This trial was registered at www.clinicaltrials.gov as #NCT04746092.
Keywords: BNT162b2; CLL; COVID-19 vaccine; NEOPLASIA/Lymphoid leukemias; antibody response.
© 2021 by The American Society of Hematology.
Figures


Comment in
-
Vaccination against COVID-19: a challenge in CLL.Blood. 2021 Jun 10;137(23):3153-3154. doi: 10.1182/blood.2021011935. Blood. 2021. PMID: 34110406 Free PMC article. No abstract available.
Comment on
-
Efficacy of COVID-19 vaccine in patients with CLL.Blood. 2021 Jun 10;137(23):3311. doi: 10.1182/blood.2021012262. Blood. 2021. PMID: 34110398 Free PMC article. No abstract available.
References
-
- Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia [published correction appears in Expert Rev Hematol. 2018;11(1):ix]. Expert Rev Hematol. 2018;11(1):57-70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

